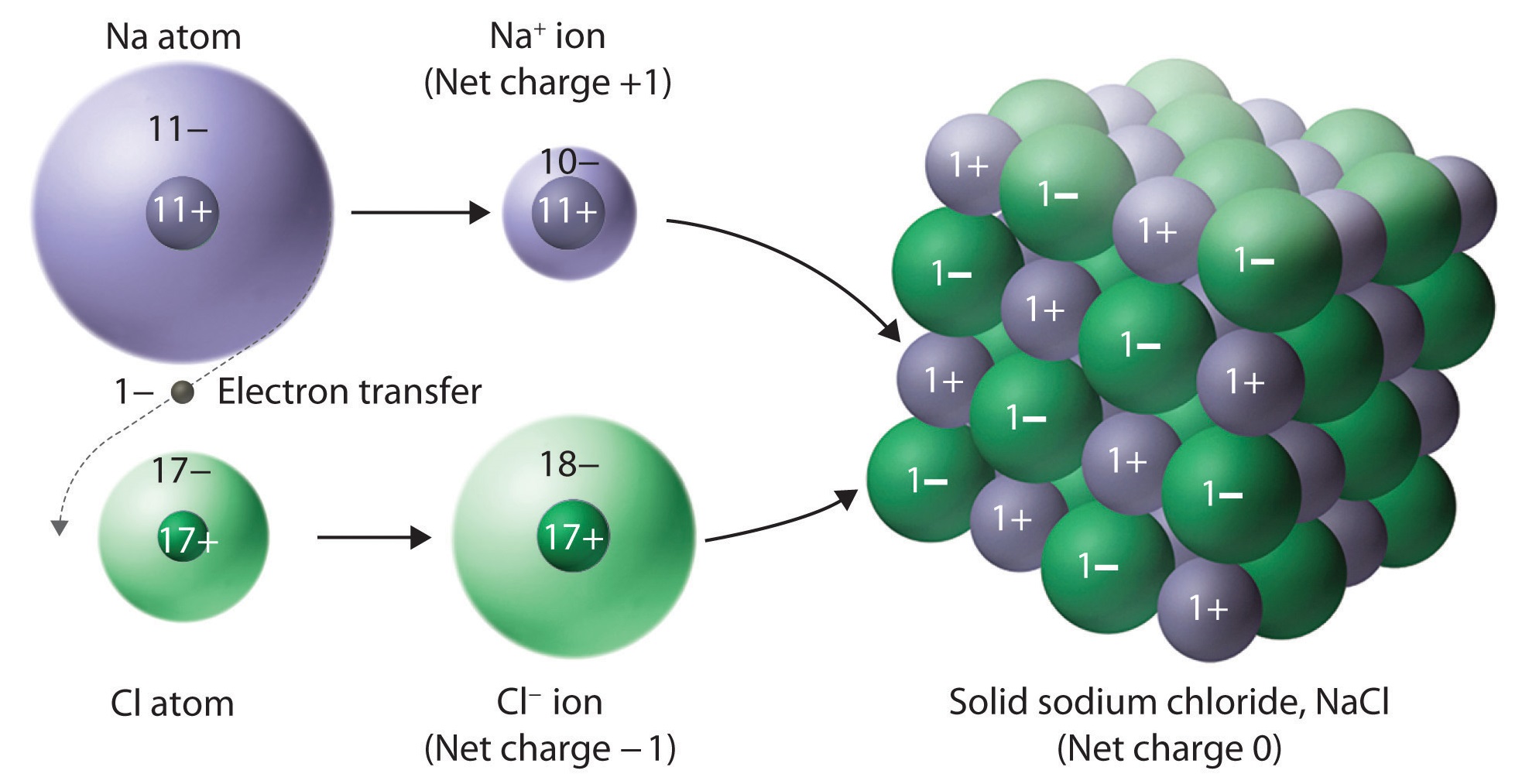
Why Ionic Compounds Hard But Brittle . When an external force is applied, it can shift the layers. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are strong and hard because of the strong. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle because they have a crystal lattice structure. Your starting assumption that ionic compounds are frequently brittle is misleading. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from.
from socratic.org
Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. When an external force is applied, it can shift the layers. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are not unusual in being brittle. Ionic compounds are strong and hard because of the strong. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from. Your starting assumption that ionic compounds are frequently brittle is misleading.
What structural units make up ionic solids? Socratic
Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. When an external force is applied, it can shift the layers. Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from. Ionic compounds are strong and hard because of the strong. Ionic compounds are not unusual in being brittle.
From www.slideserve.com
PPT Ionic and Covalent bonds PowerPoint Presentation, free download Why Ionic Compounds Hard But Brittle Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are brittle because they. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. When an external force is applied, it can shift the layers. Ionic compounds are brittle because they have a crystal lattice structure. Find. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID1083550 Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. When an external force is applied, it can shift the layers. Explain why most ionic compounds are strong. Why Ionic Compounds Hard But Brittle.
From socratic.org
What structural units make up ionic solids? Socratic Why Ionic Compounds Hard But Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. It takes a large amount of mechanical force, such as striking a. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Chemical Bonding. ppt download Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are strong and hard because of the strong. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. When an. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I PART 2 PowerPoint Presentation Why Ionic Compounds Hard But Brittle Ionic compounds are brittle because they have a crystal lattice structure. Your starting assumption that ionic compounds are frequently brittle is misleading. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. When an. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT Unit 7 PowerPoint Presentation, free download ID2642688 Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. Ionic compounds are not unusual in being brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic. Why Ionic Compounds Hard But Brittle.
From cabinet.matttroy.net
Is Table Salt A Molecular Compound Matttroy Why Ionic Compounds Hard But Brittle Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds.. Why Ionic Compounds Hard But Brittle.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Why Ionic Compounds Hard But Brittle Ionic compounds are brittle because they have a crystal lattice structure. Find out how ionic compounds differ from. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are strong and hard because of the strong. It takes a large amount of mechanical force, such as striking a crystal. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Why Ionic Compounds Hard But Brittle Ionic compounds are not unusual in being brittle. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from. Your starting assumption that ionic compounds are frequently brittle is misleading. Explain why most ionic compounds are strong and hard, yet. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
So, what makes a bond ionic? ppt download Why Ionic Compounds Hard But Brittle Find out how ionic compounds differ from. Ionic compounds are brittle because they have a crystal lattice structure. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are strong and hard because of the strong. Ionic compounds are not. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Compound A compound made up of one or more positive metal ions Why Ionic Compounds Hard But Brittle Ionic compounds are generally hard, but brittle. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Find out how ionic compounds differ from. Explain why most ionic compounds are strong and hard, yet brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard,. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Why Ionic Compounds Hard But Brittle Ionic compounds are strong and hard because of the strong. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from.. Why Ionic Compounds Hard But Brittle.
From slidetodoc.com
Compounds Ionic Compounds made by combination of metal Why Ionic Compounds Hard But Brittle Ionic compounds are brittle because they have a crystal lattice structure. Find out how ionic compounds differ from. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are not unusual in being. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Why Ionic Compounds Hard But Brittle Ionic compounds are not unusual in being brittle. When an external force is applied, it can shift the layers. Ionic compounds are generally hard, but brittle. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large. Why Ionic Compounds Hard But Brittle.
From www.doubtnut.com
Explain why ionic compounds are hard and brittle? Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are brittle because they have a crystal lattice structure. When an external force is applied, it can shift the layers. Ionic compounds are strong and hard because of the strong. Ionic compounds are not unusual. Why Ionic Compounds Hard But Brittle.
From www.toppr.com
Explanli WIIY Ionic solids are hard and brittle ugter Why Ionic Compounds Hard But Brittle Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. When an external force is applied, it can shift the layers. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Why Ionic Compounds Hard But Brittle.
From www.youtube.com
GCSE Chemistry 19 Why do Ionic Compounds have High Melting Points Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Find out how ionic compounds differ from. Ionic compounds are strong and hard because of the strong. Ionic. Why Ionic Compounds Hard But Brittle.
From brainly.com
Which property is a characteristic of an ionic compound? low melting Why Ionic Compounds Hard But Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are brittle because they have a crystal lattice structure. When an external force is applied, it can shift the layers. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. Find out how ionic compounds. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Compounds Ch.6 & ppt download Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Explain why most ionic compounds are strong and hard, yet brittle. When an external force is applied, it can shift the layers. Ionic compounds are generally hard, but brittle. Find. Why Ionic Compounds Hard But Brittle.
From favpng.com
Ionic Compound Sodium Chloride Electrical Conductivity Water, PNG Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Find out how ionic compounds differ from. Ionic compounds are not unusual in being brittle. When an external force is applied, it can shift the layers. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong.. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Bonding. ppt download Why Ionic Compounds Hard But Brittle Ionic compounds are not unusual in being brittle. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are strong and hard because of the strong. Ionic compounds are brittle because they have a crystal lattice structure. Find out how ionic compounds differ from.. Why Ionic Compounds Hard But Brittle.
From slidetodoc.com
Chemistry Lesson 1 Ionic Compounds Ionic Bonds l Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. Find out how ionic compounds differ from. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds.. Why Ionic Compounds Hard But Brittle.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Why Ionic Compounds Hard But Brittle When an external force is applied, it can shift the layers. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are generally hard, but brittle. Learn how ionic bonds are formed by large. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
WRITING AND NAMING IONIC COMPOUNDS ppt download Why Ionic Compounds Hard But Brittle Ionic compounds are not unusual in being brittle. Ionic compounds are brittle because they have a crystal lattice structure. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. Explain why most ionic compounds are strong and hard, yet brittle. Learn how ionic bonds are formed. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT BONDING & NOMENCLATURE PowerPoint Presentation, free download Why Ionic Compounds Hard But Brittle Ionic compounds are generally hard, but brittle. Ionic compounds are not unusual in being brittle. Explain why most ionic compounds are strong and hard, yet brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of ions to. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are generally hard, but brittle. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Explain why most ionic compounds are. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Compounds Chapter ppt download Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. Find out how ionic compounds differ from. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Bonding/Naming. ppt download Why Ionic Compounds Hard But Brittle Your starting assumption that ionic compounds are frequently brittle is misleading. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Find out how. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic Compounds and Solutions ppt download Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are not unusual in being brittle. Ionic compounds are strong and hard because of the strong. When an external force is applied, it can shift the layers. Find out how. Why Ionic Compounds Hard But Brittle.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Why Ionic Compounds Hard But Brittle When an external force is applied, it can shift the layers. Find out how ionic compounds differ from. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are strong and hard because of the strong. It takes a large amount of mechanical force, such as striking a crystal with a hammer, to force one layer of. Why Ionic Compounds Hard But Brittle.
From www.youtube.com
Why are ionic compounds hard and brittle? YouTube Why Ionic Compounds Hard But Brittle Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. Ionic compounds are brittle because they have a crystal lattice structure. Ionic compounds are generally hard, but brittle. Ionic compounds are strong and hard because of the strong. Your starting assumption that ionic compounds are frequently brittle is misleading. Find out how. Why Ionic Compounds Hard But Brittle.
From www.youtube.com
Why ionic solids are brittle? YouTube Why Ionic Compounds Hard But Brittle Find out how ionic compounds differ from. Explain why most ionic compounds are strong and hard, yet brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. It takes a large amount of mechanical force, such as striking a. Why Ionic Compounds Hard But Brittle.
From slideplayer.com
Ionic bonding. ppt download Why Ionic Compounds Hard But Brittle It takes a large amount of mechanical force, such as striking a crystal with a hammer, to. Your starting assumption that ionic compounds are frequently brittle is misleading. Find out how ionic compounds differ from. Learn how ionic bonds are formed by large electronegativity differences and how they affect the properties of ionic compounds. Ionic compounds are generally hard, but. Why Ionic Compounds Hard But Brittle.
From www.slideserve.com
PPT Properties of Substances PowerPoint Presentation, free download Why Ionic Compounds Hard But Brittle Explain why most ionic compounds are strong and hard, yet brittle. Ionic compounds are generally hard, but brittle. Your starting assumption that ionic compounds are frequently brittle is misleading. When an external force is applied, it can shift the layers. Find out how ionic compounds differ from. Ionic compounds are brittle because they have a crystal lattice structure. It takes. Why Ionic Compounds Hard But Brittle.