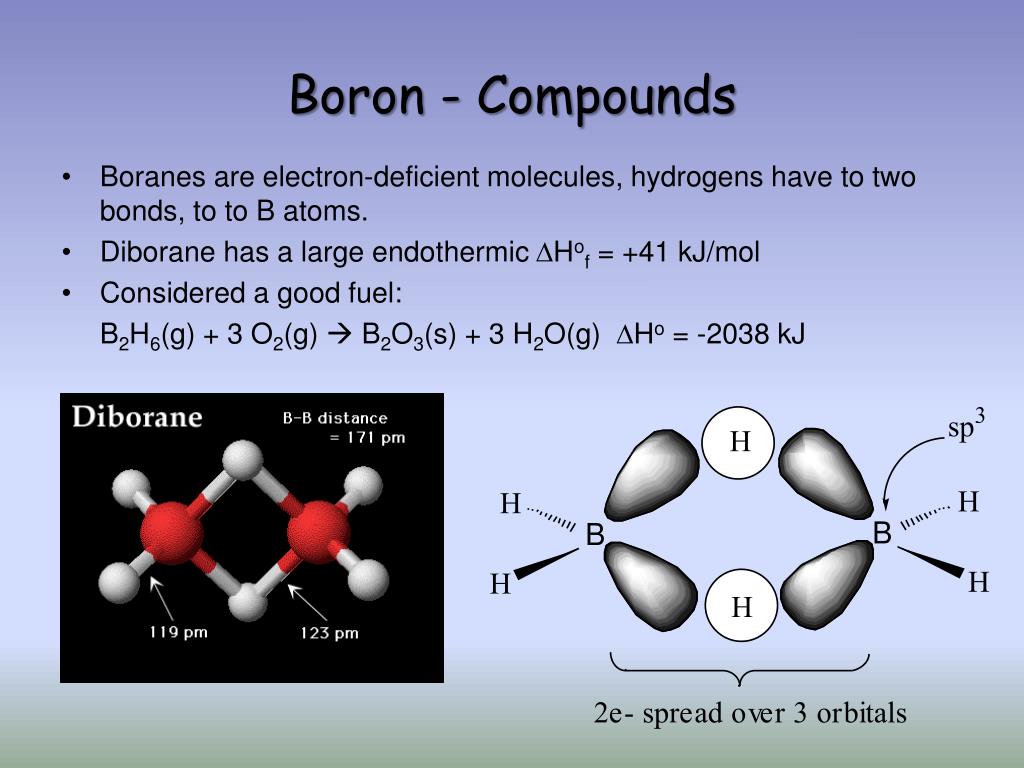
Can Boron Form Ionic Compounds . Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron can form ions but there is some fine print. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Thus, following fajan's rule, boron forms covalent compounds. You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The sum of the first three.
from www.slideserve.com
The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron can form ions but there is some fine print. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. You won't get monatomic cations like the metals below it. The sum of the first three. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Thus, following fajan's rule, boron forms covalent compounds.
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free
Can Boron Form Ionic Compounds Boron can form ions but there is some fine print. You won't get monatomic cations like the metals below it. Thus, following fajan's rule, boron forms covalent compounds. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of the first three. Boron can form ions but there is some fine print. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms.
From purehealthsolutions.com.au
Ionic Boron 500ml Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The sum of the first three. Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and. Can Boron Form Ionic Compounds.
From www.researchgate.net
Boronhydrogen speciation and influence on ionic conductivity of Can Boron Form Ionic Compounds The sum of the first three. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. You won't get monatomic cations like the metals below it. Thus, following fajan's rule, boron forms covalent compounds. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Can Boron Form Ionic Compounds Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Thus, following fajan's rule, boron forms covalent compounds. The sum of the first three. The size of bx3+ b x 3. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Can Boron Form Ionic Compounds The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The sum of the first three. Thus, following fajan's rule, boron forms covalent compounds. Ionic bonds are created when there is. Can Boron Form Ionic Compounds.
From www.youtube.com
B 3+ Electron Configuration (Boron Ion) YouTube Can Boron Form Ionic Compounds Thus, following fajan's rule, boron forms covalent compounds. The sum of the first three. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. You won't get monatomic cations like the metals below it. Ionic bonds are created when there is big enough difference between attraction of valence electrons. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT Naming Ionic Compounds PowerPoint Presentation, free download Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The size of bx3+ b x 3 + ions,. Can Boron Form Ionic Compounds.
From lessonmagicbastides.z14.web.core.windows.net
What Is The Formation Of Ions Can Boron Form Ionic Compounds Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron can form ions but there is some fine print. You won't get monatomic cations like the metals below it. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of. Can Boron Form Ionic Compounds.
From www.youtube.com
Examples of Ionic Compoiunds YouTube Can Boron Form Ionic Compounds The sum of the first three. You won't get monatomic cations like the metals below it. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron is found in nature on earth. Can Boron Form Ionic Compounds.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The sum of the first three. Thus, following fajan's rule, boron forms covalent compounds. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. With its high ionization energy, low electron affinity,. Can Boron Form Ionic Compounds.
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of the first three. Boron can form ions but there is some fine print. Thus, following fajan's rule, boron. Can Boron Form Ionic Compounds.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Thus, following fajan's. Can Boron Form Ionic Compounds.
From courses.lumenlearning.com
Boron Boundless Chemistry Can Boron Form Ionic Compounds Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. The sum of the first three. Thus, following fajan's rule, boron forms covalent compounds. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. With its high ionization energy, low electron affinity, low. Can Boron Form Ionic Compounds.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Can Boron Form Ionic Compounds The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The sum of the first three. Ionic bonds are created when there is big enough difference between attraction of valence electrons. Can Boron Form Ionic Compounds.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Can Boron Form Ionic Compounds Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. You won't get monatomic cations like the metals below it. Thus, following fajan's. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT THE GROUP 13 ELEMENTS PowerPoint Presentation, free download ID Can Boron Form Ionic Compounds Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. With its high ionization energy, low electron affinity, low electronegativity, and small size,. Can Boron Form Ionic Compounds.
From saylordotorg.github.io
Naming Ionic Compounds Can Boron Form Ionic Compounds With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Boron can form ions but there is some fine print. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. The sum of the first three. The size of. Can Boron Form Ionic Compounds.
From www.youtube.com
Boron Family Reaction with Oxygen YouTube Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. You. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Can Boron Form Ionic Compounds Boron can form ions but there is some fine print. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Thus, following fajan's rule, boron forms covalent compounds. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The. Can Boron Form Ionic Compounds.
From chemistry.about.com
Examples of Ionic Bonds and Ionic Compounds Can Boron Form Ionic Compounds The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. You won't get monatomic cations like the metals below it. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. With its high ionization energy, low electron affinity, low electronegativity, and. Can Boron Form Ionic Compounds.
From medium.com
What is Boron? Periodic Table Elements Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The sum of the first three. Ionic bonds are created. Can Boron Form Ionic Compounds.
From hxeeheshc.blob.core.windows.net
Does Boron Not Form Ionic Compounds at Allen Birch blog Can Boron Form Ionic Compounds With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements.. Can Boron Form Ionic Compounds.
From study.com
Chemical Formula for Ionic Compound Binary & Polyatomic Lesson Can Boron Form Ionic Compounds Thus, following fajan's rule, boron forms covalent compounds. The sum of the first three. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Boron can form ions but there is some. Can Boron Form Ionic Compounds.
From www.youtube.com
Is BBr3 (Boron tribromide ) Ionic or Covalent/Molecular? YouTube Can Boron Form Ionic Compounds The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. The sum of the first three. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated. Can Boron Form Ionic Compounds.
From www.youtube.com
Lewis Dot Structure for BoronHow do you draw the Lewis Dot structure Can Boron Form Ionic Compounds The sum of the first three. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. You won't get monatomic cations like the metals below it. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Ionic bonds are created. Can Boron Form Ionic Compounds.
From www.britannica.com
boron Properties, Uses, & Facts Britannica Can Boron Form Ionic Compounds With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Thus, following fajan's rule, boron forms covalent compounds. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of the first three. Boron can form ions but there. Can Boron Form Ionic Compounds.
From phys.org
Unprecedented formation of a boronboron covalent bond opens a new Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. With. Can Boron Form Ionic Compounds.
From www.youtube.com
SOME IMPORTANT COMPOUNDS OF BORON YouTube Can Boron Form Ionic Compounds The sum of the first three. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron. Can Boron Form Ionic Compounds.
From mavink.com
5 Examples Of Ionic Compounds Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of. Can Boron Form Ionic Compounds.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Can Boron Form Ionic Compounds Thus, following fajan's rule, boron forms covalent compounds. The sum of the first three. You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. The size of bx3+ b x 3 + ions, is very small and the ion. Can Boron Form Ionic Compounds.
From www.britannica.com
Boron group element Trihalides, Properties, Uses Britannica Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron is found in nature on earth almost entirely as various. Can Boron Form Ionic Compounds.
From h-o-m-e.org
Boron Trihydride (BH3) The Exception to the Octet Rule Can Boron Form Ionic Compounds The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Thus, following fajan's. Can Boron Form Ionic Compounds.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Can Boron Form Ionic Compounds Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Boron can form ions but there is some fine print. You won't get monatomic cations like the metals below. Can Boron Form Ionic Compounds.
From www.britannica.com
Boron group element Properties & Facts Britannica Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. With its high ionization energy, low electron affinity, low electronegativity, and small size, however, boron does not form a metallic lattice. Thus, following fajan's rule, boron forms covalent compounds. Boron is found in nature on earth almost entirely as various oxides of b (iii), often associated with other elements. Ionic. Can Boron Form Ionic Compounds.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube Can Boron Form Ionic Compounds You won't get monatomic cations like the metals below it. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective atoms. Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of. Can Boron Form Ionic Compounds.
From boron-staidans.weebly.com
Bonding Boron Can Boron Form Ionic Compounds Boron can form ions but there is some fine print. The size of bx3+ b x 3 + ions, is very small and the ion has high charge density. The sum of the first three. Thus, following fajan's rule, boron forms covalent compounds. Ionic bonds are created when there is big enough difference between attraction of valence electrons by respective. Can Boron Form Ionic Compounds.