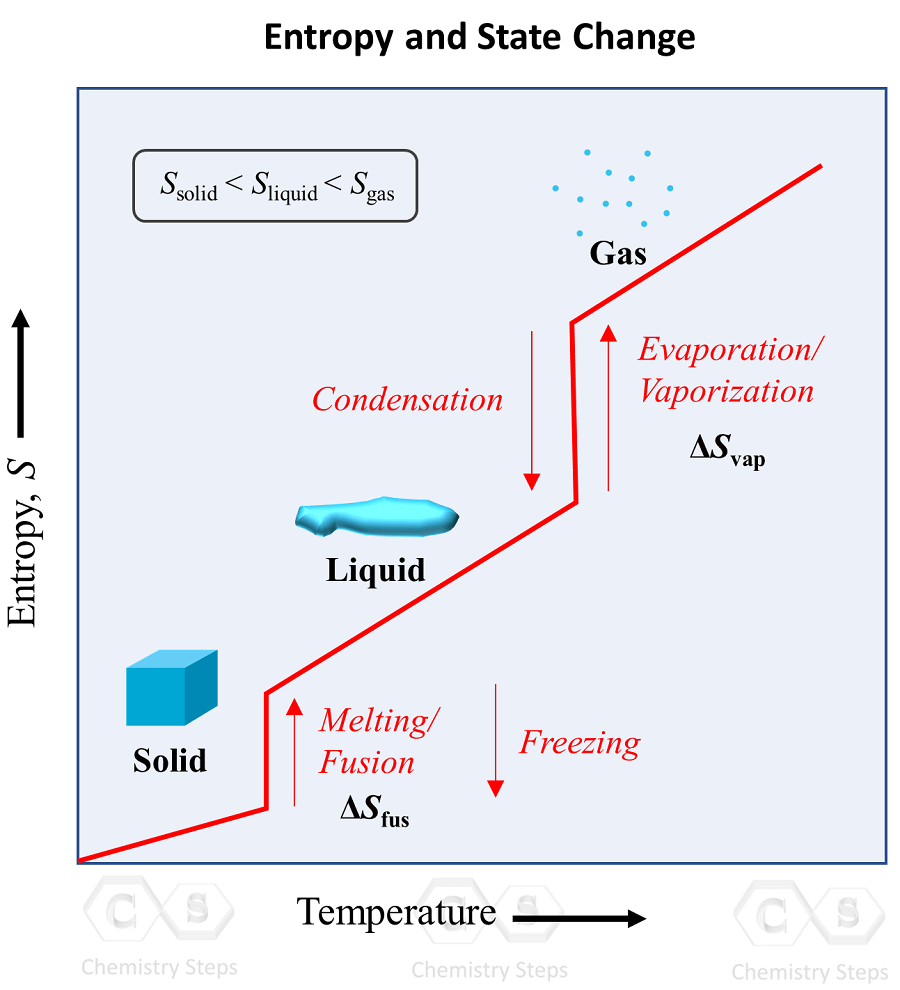
Solid To Liquid Diagram . When a solid like my ice lolly is heated, it melts to become a liquid. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Which phase, solid or liquid, has the highest density in this system? There are also two important points on the diagram, the triple point and the critical point. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,.
from general.chemistrysteps.com
There are also two important points on the diagram, the triple point and the critical point. Which phase, solid or liquid, has the highest density in this system? And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). When a solid like my ice lolly is heated, it melts to become a liquid.
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps
Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Which phase, solid or liquid, has the highest density in this system? There are also two important points on the diagram, the triple point and the critical point. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. When a solid like my ice lolly is heated, it melts to become a liquid. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid To Liquid Diagram Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even. Solid To Liquid Diagram.
From www.thoughtco.com
List of Phase Changes Between States of Matter Solid To Liquid Diagram Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. There are also two important points on the diagram, the triple point and the critical point. Which phase, solid or liquid, has the highest density in this system? The blue divides the liquid and gas phases, represents vaporization (liquid to gas). Solid To Liquid Diagram.
From schematicviciosinfin17.z22.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid To Liquid Diagram The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Which phase, solid or liquid, has the highest density in this system? When a solid like my ice lolly is heated,. Solid To Liquid Diagram.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Which phase, solid or liquid, has the highest density in this system? When a solid like my ice lolly is heated, it. Solid To Liquid Diagram.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? There are also two important points on the diagram, the triple point and the critical point. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. And if you heat up a liquid even more, it’ll evaporate to become. Solid To Liquid Diagram.
From www.britannica.com
solid Definition & Facts Britannica Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). There are. Solid To Liquid Diagram.
From www.youtube.com
Solidliquid phase diagrams YouTube Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Which phase, solid. Solid To Liquid Diagram.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Diagram There are also two important points on the diagram, the triple point and the critical point. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue divides the liquid. Solid To Liquid Diagram.
From emedia.rmit.edu.au
States of matter Learning Lab Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? There are also two important points on the diagram, the triple point and the critical point. When a solid like my ice lolly is heated, it melts to become a liquid. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing. Solid To Liquid Diagram.
From thehungryjpeg.com
Different states of matter solid, liquid, gas vector diagram By Solid To Liquid Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Which phase, solid or liquid, has the highest density in this system? When a solid like my ice lolly is heated, it melts to become a liquid. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation. Solid To Liquid Diagram.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation, free Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Phase transition. Solid To Liquid Diagram.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6457347 Solid To Liquid Diagram Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. The green line divides. Solid To Liquid Diagram.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram Solid To Liquid Diagram When a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). There are also two important points. Solid To Liquid Diagram.
From guidewiringmikrons.z14.web.core.windows.net
Phase Diagram Melting Point Solid To Liquid Diagram When a solid like my ice lolly is heated, it melts to become a liquid. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Which phase, solid or liquid, has the highest density in this system? Phase transition is when a substance changes from a solid, liquid, or gas state. Solid To Liquid Diagram.
From mungfali.com
Solid Liquid Gas Phase Change Diagram Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Which phase, solid. Solid To Liquid Diagram.
From www.researchgate.net
Schematic diagram of the gasliquidsolid conversion (a); the Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). When a solid like my ice lolly is heated, it. Solid To Liquid Diagram.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Solid To Liquid Diagram Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. When a solid like my ice lolly is heated, it melts to become a liquid. The blue divides the liquid and gas phases,. Solid To Liquid Diagram.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? When a solid like my ice lolly is heated, it melts to become a liquid. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The green line divides the solid and liquid phases and represents melting (solid to. Solid To Liquid Diagram.
From www.oxnotes.com
States of Matter Revision Notes IGCSE Chemistry OxNotes GCSE Revision Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. When a solid like my ice lolly is heated, it melts to. Solid To Liquid Diagram.
From easyscienceforkids.com
Facts About States of Matter Easy Science For KidsEasy Science For Kids Solid To Liquid Diagram There are also two important points on the diagram, the triple point and the critical point. When a solid like my ice lolly is heated, it melts to become a liquid. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. And if you heat up a liquid even more, it’ll. Solid To Liquid Diagram.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Solid To Liquid Diagram Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. When a solid like my ice lolly is heated, it melts to become a liquid. There are also two important points on the diagram, the triple point and the critical point. Which phase, solid or liquid, has the highest density in. Solid To Liquid Diagram.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Solid To Liquid Diagram There are also two important points on the diagram, the triple point and the critical point. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Starting from the very basics it explains. Solid To Liquid Diagram.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? When a solid like my ice lolly is heated, it melts to become a liquid. The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Starting from the very basics it explains the reading of unary, binary and. Solid To Liquid Diagram.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Solid To Liquid Diagram Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. The green line divides. Solid To Liquid Diagram.
From learncheme.com
solidsolidliquidphasediagramsconceptests2 LearnChemE Solid To Liquid Diagram There are also two important points on the diagram, the triple point and the critical point. When a solid like my ice lolly is heated, it melts to become a liquid. Which phase, solid or liquid, has the highest density in this system? The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to. Solid To Liquid Diagram.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Solid To Liquid Diagram The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). When a solid like my ice lolly is heated, it melts to become a liquid. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The blue divides the liquid and gas. Solid To Liquid Diagram.
From www.snexplores.org
Explainer What are the different states of matter? Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. There are also two. Solid To Liquid Diagram.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solid To Liquid Diagram The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Phase transition. Solid To Liquid Diagram.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Solid To Liquid Diagram And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Which phase, solid or liquid, has the highest density in this system? The green line divides the solid and liquid phases and represents. Solid To Liquid Diagram.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii Solid To Liquid Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Which phase, solid or liquid, has the highest density in this system? The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). When a solid like my ice lolly is heated, it. Solid To Liquid Diagram.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Solid To Liquid Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Which phase, solid or liquid, has the highest density in this system? Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Every element and substance can transition from one phase to another. Solid To Liquid Diagram.
From www.researchgate.net
Solidliquid temperature composition phase diagram for a binary system Solid To Liquid Diagram Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). And if you heat up a liquid even more, it’ll evaporate to become a gas, also known as boiling. When a solid like. Solid To Liquid Diagram.
From courses.lumenlearning.com
The Dissolution Process Chemistry Solid To Liquid Diagram Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). The green line divides. Solid To Liquid Diagram.
From galvinconanstuart.blogspot.com
In This Phase Diagram For Water Indicate The Direction That The Solid Solid To Liquid Diagram When a solid like my ice lolly is heated, it melts to become a liquid. The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). Starting from the very basics it explains the reading of unary, binary and ternary phase diagrams, including liquidus projections,. Phase transition is when a substance changes from. Solid To Liquid Diagram.
From chemstuff.co.uk
Particle Model of Solids, Liquids and Gases Chemstuff Solid To Liquid Diagram Which phase, solid or liquid, has the highest density in this system? There are also two important points on the diagram, the triple point and the critical point. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. The green line divides the solid and liquid phases and represents melting (solid. Solid To Liquid Diagram.