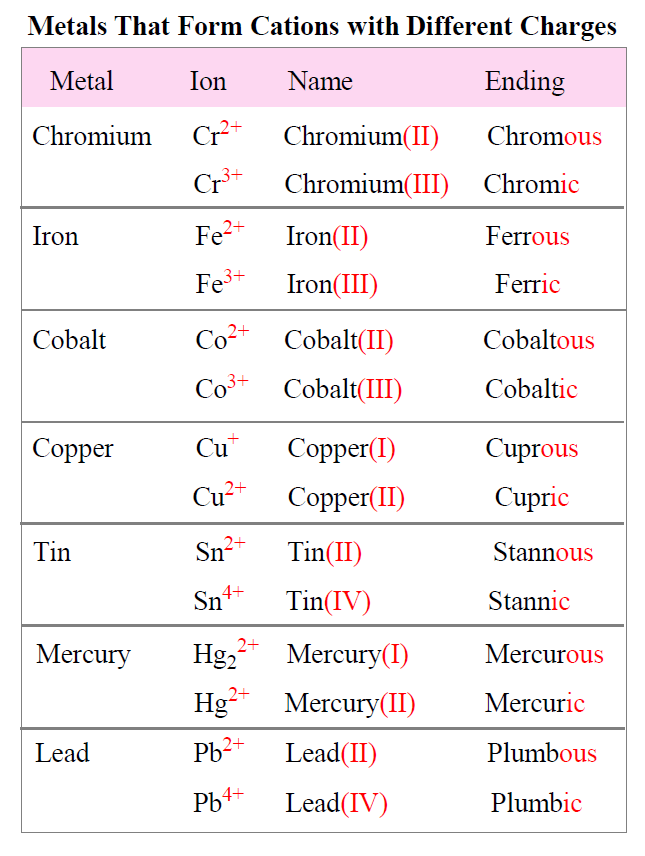
Aluminum And Chlorine Ionic Charge . Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is known. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Aluminum and chlorine form covalent bonds, not ionic bonds. 93 rows this is a table of the most common charges for atoms of the chemical elements. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; Charges predict whether an atom bonds with another atom. Predict charges (using the modified bohr model) for the following elements:
from general.chemistrysteps.com
Aluminum and chlorine form covalent bonds, not ionic bonds. Memorize charges for silver and zinc; The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. This electric charge generated on the ion is known. 93 rows this is a table of the most common charges for atoms of the chemical elements. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Charges predict whether an atom bonds with another atom. Predict charges (using the modified bohr model) for the following elements:
Naming Ionic Compounds Chemistry Steps
Aluminum And Chlorine Ionic Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Charges predict whether an atom bonds with another atom. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. This electric charge generated on the ion is known. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Memorize charges for silver and zinc; Predict charges (using the modified bohr model) for the following elements: 93 rows this is a table of the most common charges for atoms of the chemical elements. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Aluminum and chlorine form covalent bonds, not ionic bonds.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminum And Chlorine Ionic Charge Charges predict whether an atom bonds with another atom. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. This electric charge generated on the ion is known. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Memorize charges for. Aluminum And Chlorine Ionic Charge.
From topblogtenz.com
Is AlCl3 ionic or covalent or both? What type of bond in Aluminium Aluminum And Chlorine Ionic Charge Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; When the atom loses or gains one or more electrons, the electric charge is generated (and an ion. Aluminum And Chlorine Ionic Charge.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Aluminum And Chlorine Ionic Charge 93 rows this is a table of the most common charges for atoms of the chemical elements. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Predict charges (using the modified bohr model) for the following elements: Three fluorine 1− ions are needed to balance the 3+ charge. Aluminum And Chlorine Ionic Charge.
From courses.lumenlearning.com
Lewis Symbols and Structures Introductory Chemistry Lecture & Lab Aluminum And Chlorine Ionic Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Predict charges (using the modified bohr model) for the following elements: This electric charge generated on the ion is known. Memorize charges for silver and zinc; 93 rows this is a table of the most common. Aluminum And Chlorine Ionic Charge.
From slideplayer.com
Dry Lab Nomenclature exercise ppt download Aluminum And Chlorine Ionic Charge Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. This electric charge generated on the ion is known. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). The formula of an ionic compound represents the simplest ratio of the numbers of ions. Aluminum And Chlorine Ionic Charge.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Aluminum And Chlorine Ionic Charge Aluminum and chlorine form covalent bonds, not ionic bonds. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; Charges predict whether an atom bonds with another atom. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has. Aluminum And Chlorine Ionic Charge.
From www.edplace.com
Understand Ionic Bonding Worksheet EdPlace Aluminum And Chlorine Ionic Charge Predict charges (using the modified bohr model) for the following elements: Charges predict whether an atom bonds with another atom. Memorize charges for silver and zinc; Phosphorus, nitrogen, sulfur, oxygen, and aluminum. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Three fluorine 1− ions. Aluminum And Chlorine Ionic Charge.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Aluminum And Chlorine Ionic Charge This electric charge generated on the ion is known. Aluminum and chlorine form covalent bonds, not ionic bonds. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Charges predict whether an atom bonds with another atom.. Aluminum And Chlorine Ionic Charge.
From www.slideshare.net
Ionic bonding Aluminum And Chlorine Ionic Charge Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Charges predict whether an atom bonds with another atom. Predict charges (using the modified bohr model) for the. Aluminum And Chlorine Ionic Charge.
From www.slideserve.com
PPT Ionic bonding, Lewis Dot Diagrams PowerPoint Presentation, free Aluminum And Chlorine Ionic Charge Charges predict whether an atom bonds with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Memorize charges for silver and zinc; Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Phosphorus, nitrogen, sulfur, oxygen,. Aluminum And Chlorine Ionic Charge.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum And Chlorine Ionic Charge Aluminum and chlorine form covalent bonds, not ionic bonds. Predict charges (using the modified bohr model) for the following elements: This electric charge generated on the ion is known. 93 rows this is a table of the most common charges for atoms of the chemical elements. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine. Aluminum And Chlorine Ionic Charge.
From material-properties.org
Aluminium and Chlorine Comparison Properties Material Properties Aluminum And Chlorine Ionic Charge The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Aluminum and chlorine form covalent bonds, not ionic bonds. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Memorize charges for silver and zinc;. Aluminum And Chlorine Ionic Charge.
From www.pinterest.ph
the names and symbols of different types of hydrogens, which are Aluminum And Chlorine Ionic Charge Predict charges (using the modified bohr model) for the following elements: Memorize charges for silver and zinc; Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Charges predict whether an atom bonds with another atom. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. This electric charge generated on the ion is known. The formula of an. Aluminum And Chlorine Ionic Charge.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Aluminum And Chlorine Ionic Charge This electric charge generated on the ion is known. Aluminum and chlorine form covalent bonds, not ionic bonds. Memorize charges for silver and zinc; Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Aluminum And Chlorine Ionic Charge.
From www.anyrgb.com
Aluminium chloride, ionic Compound, Valence electron, Ionic Bonding Aluminum And Chlorine Ionic Charge 93 rows this is a table of the most common charges for atoms of the chemical elements. Aluminum and chlorine form covalent bonds, not ionic bonds. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Predict charges (using the modified bohr model) for the following elements: The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to. Aluminum And Chlorine Ionic Charge.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Aluminum General Wiring Diagram Aluminum And Chlorine Ionic Charge Charges predict whether an atom bonds with another atom. Predict charges (using the modified bohr model) for the following elements: Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Three fluorine 1− ions are needed to balance the 3+ charge on the. Aluminum And Chlorine Ionic Charge.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum And Chlorine Ionic Charge Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Predict charges (using the modified bohr model) for the following elements: The formula of an ionic compound represents the simplest ratio of the numbers of. Aluminum And Chlorine Ionic Charge.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Aluminum And Chlorine Ionic Charge The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Aluminum and chlorine form covalent bonds, not ionic bonds. Memorize charges for silver and zinc; 93 rows this is a table of the most common charges for atoms of the chemical elements. Between the aluminum and. Aluminum And Chlorine Ionic Charge.
From brainly.in
Ionic bond of aluminium chloride with diagram Brainly.in Aluminum And Chlorine Ionic Charge Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion.. Aluminum And Chlorine Ionic Charge.
From www.slideserve.com
PPT A Case Study on Covalent Bonding PowerPoint Presentation, free Aluminum And Chlorine Ionic Charge Memorize charges for silver and zinc; The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Predict charges (using the modified bohr model) for the following elements: Aluminum and chlorine form covalent. Aluminum And Chlorine Ionic Charge.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the Aluminum And Chlorine Ionic Charge This electric charge generated on the ion is known. Aluminum and chlorine form covalent bonds, not ionic bonds. 93 rows this is a table of the most common charges for atoms of the chemical elements. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The formula of an ionic compound represents the simplest ratio. Aluminum And Chlorine Ionic Charge.
From slideplayer.com
Ionic Compounds Naming and Writing Formulas ppt download Aluminum And Chlorine Ionic Charge 93 rows this is a table of the most common charges for atoms of the chemical elements. Aluminum and chlorine form covalent bonds, not ionic bonds. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). When the atom loses or gains one or more electrons, the electric charge. Aluminum And Chlorine Ionic Charge.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube Aluminum And Chlorine Ionic Charge Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Memorize charges for silver and zinc; The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When the atom loses or gains one or more. Aluminum And Chlorine Ionic Charge.
From www.slideserve.com
PPT Unit 6 Test Review PowerPoint Presentation, free download ID Aluminum And Chlorine Ionic Charge Memorize charges for silver and zinc; Aluminum and chlorine form covalent bonds, not ionic bonds. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Phosphorus, nitrogen, sulfur, oxygen, and aluminum. This. Aluminum And Chlorine Ionic Charge.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Aluminum And Chlorine Ionic Charge Predict charges (using the modified bohr model) for the following elements: Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Memorize charges for silver and zinc; The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The aluminum ion has a 3+ charge, while the fluoride ion formed by. Aluminum And Chlorine Ionic Charge.
From www.youtube.com
How to Balance AlCl3 = Al + Cl2 (Aluminum chloride by Aluminum And Chlorine Ionic Charge Memorize charges for silver and zinc; Predict charges (using the modified bohr model) for the following elements: Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Phosphorus, nitrogen, sulfur, oxygen, and aluminum. Aluminum and chlorine form covalent bonds, not ionic bonds. Charges predict whether an atom bonds with. Aluminum And Chlorine Ionic Charge.
From guidelistpstrapanning.z14.web.core.windows.net
Diagram Ionic Bond Aluminum And Chlorine Ionic Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. This electric charge generated on the ion is known. Aluminum and chlorine form covalent bonds, not ionic bonds. Phosphorus, nitrogen, sulfur, oxygen, and aluminum. When the atom loses or gains one or more electrons, the electric. Aluminum And Chlorine Ionic Charge.
From www.slideshare.net
Ionic bonding Aluminum And Chlorine Ionic Charge Phosphorus, nitrogen, sulfur, oxygen, and aluminum. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Charges predict whether an atom bonds with another atom. Predict charges (using the modified bohr model). Aluminum And Chlorine Ionic Charge.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Aluminum And Chlorine Ionic Charge Phosphorus, nitrogen, sulfur, oxygen, and aluminum. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Memorize charges for silver and zinc; Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). This electric charge generated on the ion is known.. Aluminum And Chlorine Ionic Charge.
From www.numerade.com
SOLVEDWrite a formula for the ionic compound that forms between each Aluminum And Chlorine Ionic Charge Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. Memorize charges for silver and zinc; Charges predict whether an atom bonds with another atom. This electric charge generated on the ion is known. Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond).. Aluminum And Chlorine Ionic Charge.
From slideplayer.com
Ionic Compounds Formulas and Naming ppt download Aluminum And Chlorine Ionic Charge Memorize charges for silver and zinc; Aluminum and chlorine form covalent bonds, not ionic bonds. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Predict charges (using the modified bohr model) for the following elements: When the atom loses or gains one or more electrons, the electric charge is generated (and. Aluminum And Chlorine Ionic Charge.
From en.wikipedia.org
Ionic bonding Wikipedia Aluminum And Chlorine Ionic Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Aluminum and chlorine form covalent bonds, not ionic bonds. Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The aluminum ion has a 3+ charge, while the fluoride ion formed. Aluminum And Chlorine Ionic Charge.
From chemistryguru.com.sg
Why is Aluminium Chloride a Simple Covalent Compound Aluminum And Chlorine Ionic Charge Between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence a polar bond). Three fluorine 1− ions are needed to balance the 3+ charge on the aluminum ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative.. Aluminum And Chlorine Ionic Charge.
From socratic.org
What structural units make up ionic solids? Socratic Aluminum And Chlorine Ionic Charge The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Memorize charges for silver and zinc; Predict charges (using the modified bohr model) for the following elements: 93 rows this is a table of the most common charges for atoms of the chemical elements. Aluminum and chlorine form covalent bonds, not ionic. Aluminum And Chlorine Ionic Charge.
From circuitmanualelfriede.z4.web.core.windows.net
Cl Lewis Structure Diagram Aluminum And Chlorine Ionic Charge Charges predict whether an atom bonds with another atom. The aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. This electric charge generated on the ion is known. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative.. Aluminum And Chlorine Ionic Charge.