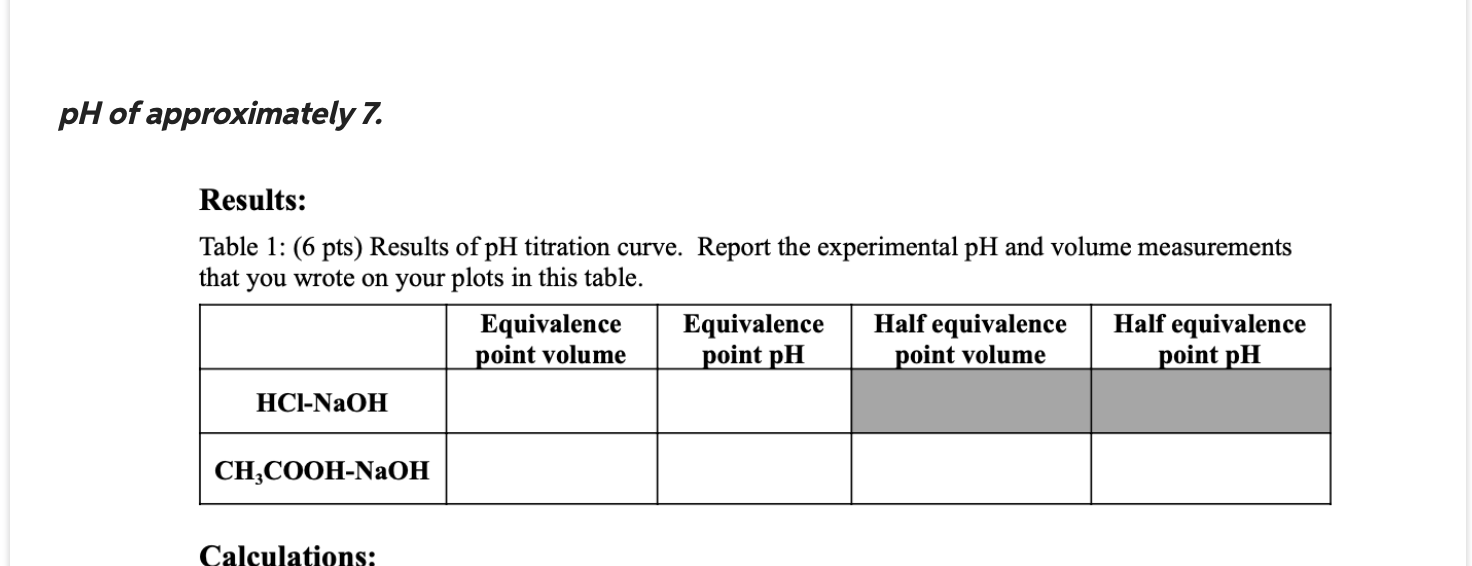
Titration Curve Ch3Cooh Naoh . Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Hcl by titration with naoh (known concentration) and using the titration curve. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Before the addition of the base, the acid solution has a high. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. When hn o3(aq) is titrated with. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base.
from www.chegg.com
Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 Hcl by titration with naoh (known concentration) and using the titration curve. When hn o3(aq) is titrated with. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Before the addition of the base, the acid solution has a high. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base.
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration
Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Before the addition of the base, the acid solution has a high. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 When hn o3(aq) is titrated with. Hcl by titration with naoh (known concentration) and using the titration curve. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh.
From mungfali.com
Half Equivalence Point On Titration Curve Titration Curve Ch3Cooh Naoh Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and. Titration Curve Ch3Cooh Naoh.
From www.youtube.com
DAT Titration Curve of Weak Acid Strong Base (CH3COOH and NaOH Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. Before the addition of the base, the acid solution has a high. When hn o3(aq) is titrated with. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. When solving a titration problem. Titration Curve Ch3Cooh Naoh.
From www.researchgate.net
Titration of 0.3 g etching solution (40 HNO3, 7 HF, and 30 CH3COOH Titration Curve Ch3Cooh Naoh Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m. Titration Curve Ch3Cooh Naoh.
From pubs.sciepub.com
Figure 10B. Second derivative plot of the titration of weak acid Titration Curve Ch3Cooh Naoh When hn o3(aq) is titrated with. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titration curve for the titration of 50.0 ml of 0.100 m hcl. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved points Questions 1. From the NaOHCH3COOH titration Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. Acetic acid (ch 3 cooh) is a weak acid having. Titration Curve Ch3Cooh Naoh.
From www.youtube.com
CH3COOH+NaOH=CH3COONa+H2O. balance the chemical equation Titration Curve Ch3Cooh Naoh The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and. Titration Curve Ch3Cooh Naoh.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID4273257 Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Before the addition of. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED Text Question 14 of 15 10 mL of 1.8 M acetic acid (CH3COOH Titration Curve Ch3Cooh Naoh The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 Hcl by titration with naoh (known. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED Which of the following reactions might describe the titration Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Hcl by titration with naoh (known concentration) and using the titration curve. Before the addition of the base, the acid solution has a high. When hn o3(aq) is titrated with. Acetic acid (ch 3 cooh) is a weak. Titration Curve Ch3Cooh Naoh.
From pubs.sciepub.com
Figure 9. First derivative plot of the titration of weak acid (CH3COOH Titration Curve Ch3Cooh Naoh Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Hcl by titration with naoh (known concentration) and using the titration curve.. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved The titration of 25.00 mL of 0.100 M CH3COOH with Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. The titration curves. Titration Curve Ch3Cooh Naoh.
From www.doubtnut.com
conductometric titration curve of a equimolar mixture of a HCl and HCN Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. When hn. Titration Curve Ch3Cooh Naoh.
From pubs.sciepub.com
Figure 8A. Plot of the titration of weak acid (CH3COOH= 0.1M) with Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. [chx3cooh]. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED What acid could yield the titration curve below? 14 12 10 1 12 Titration Curve Ch3Cooh Naoh Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. When hn o3(aq) is titrated with. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 The titration curves of weak acid. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. When hn o3(aq) is titrated with. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Hcl by titration with naoh (known concentration) and using the. Titration Curve Ch3Cooh Naoh.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free Titration Curve Ch3Cooh Naoh The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Before the addition of the base, the acid solution has a high. [chx3cooh]. Titration Curve Ch3Cooh Naoh.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Curve Ch3Cooh Naoh The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain.. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration Titration Curve Ch3Cooh Naoh [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. When hn o3(aq) is titrated with. Conductometric titration curve of. Titration Curve Ch3Cooh Naoh.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Ch3Cooh Naoh Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as. Titration Curve Ch3Cooh Naoh.
From www.youtube.com
Conductometric Titration "Weak acid vs Strong base" Practical Titration Curve Ch3Cooh Naoh The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. When hn o3(aq) is titrated with. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 Before the addition of the base, the acid solution has a high. Hcl by titration with naoh (known concentration) and. Titration Curve Ch3Cooh Naoh.
From www.youtube.com
Titration of CH3COOH with NaOH Part 2 YouTube Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. When hn o3(aq) is titrated with. The titration curves of weak acid titrated with strong base and weak. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED You titrated CH3COOH with NaOH and stopped the titration. On Titration Curve Ch3Cooh Naoh When hn o3(aq) is titrated with. Hcl by titration with naoh (known concentration) and using the titration curve. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. Before the addition of the base,. Titration Curve Ch3Cooh Naoh.
From www.youtube.com
P. 2. Conductometric titration CH3COOH vs NaOH YouTube Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. When hn o3(aq) is titrated with. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED Max Buffering Capacity Unanswered 3 attempts left Mark the Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and. Titration Curve Ch3Cooh Naoh.
From socratic.org
Mixing up equal volumes of 0.1 M NaOH and 0.1 M CH3COOH yields a Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are. Titration Curve Ch3Cooh Naoh.
From chart-studio.plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Curve Ch3Cooh Naoh When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. When hn o3(aq) is titrated with. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Hcl by titration with naoh (known concentration) and using the. Titration Curve Ch3Cooh Naoh.
From pubs.sciepub.com
Figure 8B. Plot of the titration of weak acid (CH3COOH= 0.1M) with Titration Curve Ch3Cooh Naoh Before the addition of the base, the acid solution has a high. Hcl by titration with naoh (known concentration) and using the titration curve. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Titration curve for the titration of 50.0 ml of 0.100 m. Titration Curve Ch3Cooh Naoh.
From chart-studio.plotly.com
Acetic Acid (CH3COOH) + Sodium Hydroxide (NaOH) Titration scatter Titration Curve Ch3Cooh Naoh Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. When hn o3(aq) is titrated with. Hcl by titration with naoh (known concentration) and using the titration curve. [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 Titration curve for the titration. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVEDSketch titration curves for the following acidbase titrations Titration Curve Ch3Cooh Naoh Before the addition of the base, the acid solution has a high. When hn o3(aq) is titrated with. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. Hcl by titration with naoh (known concentration) and using the titration curve. When solving a titration problem. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved The titration curves for HCl and CH3COOH when Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. The titration curves. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED Draw precisely the titration curve for the addition of NaOH to Titration Curve Ch3Cooh Naoh When hn o3(aq) is titrated with. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titration curve for the titration of 50.0 ml of 0.100. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved HCl NaOH Titration CurveCH3COOH NaOH Titration Titration Curve Ch3Cooh Naoh Acetic acid (ch 3 cooh) is a weak acid having a ph value of more than 6 and sodium hydroxide (naoh) is a strong base. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The titration curves of weak acid titrated with strong base and weak base. Titration Curve Ch3Cooh Naoh.
From www.chegg.com
Solved HCl NaOH Titration Curve CH3COOH NaOH Titration Titration Curve Ch3Cooh Naoh Hcl by titration with naoh (known concentration) and using the titration curve. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. Titration curve for the titration of 50.0 ml of 0.100 m hcl with 0.200 m naoh. The titration curves of weak acid titrated with strong base and weak. Titration Curve Ch3Cooh Naoh.
From byjus.com
The graph of pH during the titration of NaOH and HCl Titration Curve Ch3Cooh Naoh Before the addition of the base, the acid solution has a high. When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The titration curves of weak acid titrated with strong base and weak base titrated with strong acid are inverse of each other. Acetic acid (ch 3. Titration Curve Ch3Cooh Naoh.
From www.numerade.com
SOLVED Draw approximate titration curve for following CH3COOH(aq) is Titration Curve Ch3Cooh Naoh [chx3cooh] = 0.09 − 3v 0.09 + v mol dm − 3 When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Conductometric titration curve of an equimolar mixture of hcl and hcn with n aoh (aq) can be given as q. When hn o3(aq) is titrated with.. Titration Curve Ch3Cooh Naoh.