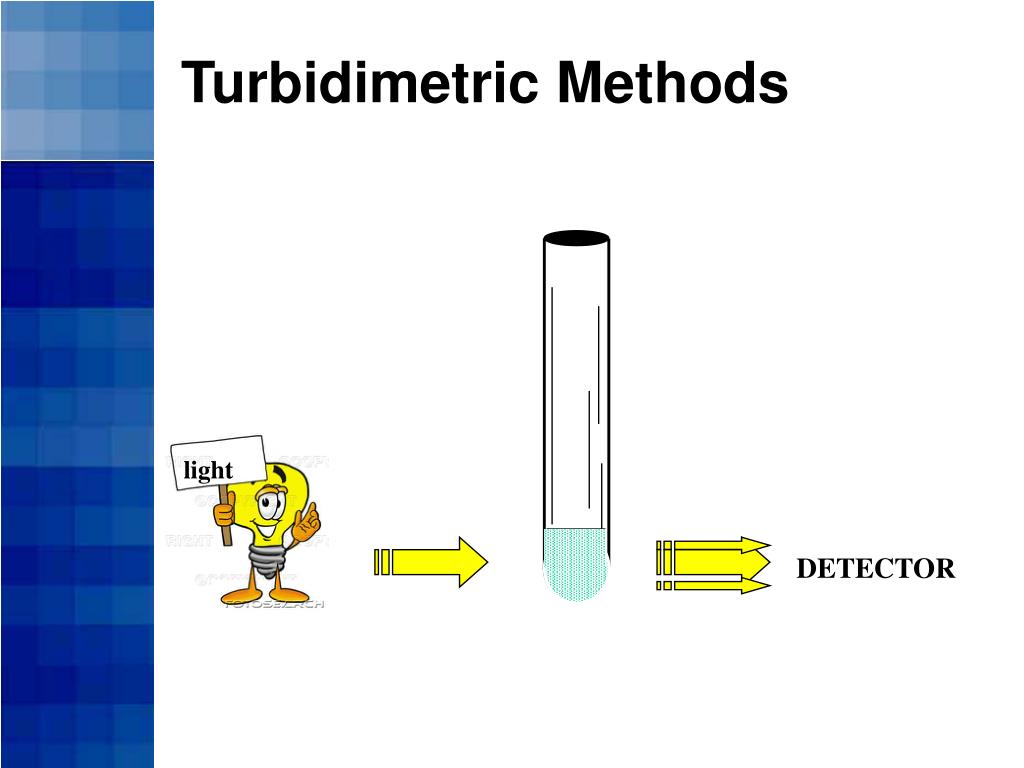
Kinetic Turbidimetric Endotoxin Testing . The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with.
from www.slideserve.com
The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are.
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint
Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the.
From www.accumedi.com
TAL (Vial, no CSE and BET water, method)_TAL Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From njlabs.com
An Overview Of The Turbidimetric Endotoxin Method NJ Labs Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.criver.com
Turbidimetric Endotoxin Test Reagent Charles River Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant. Kinetic Turbidimetric Endotoxin Testing.
From www.lonzabioscience.com.au
PYROGENT™ 5000 Bulk Turbidimetric LAL Assay, 10,000 Tests Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.indiamart.com
Lonza Turbidimetric Bacterial Endotoxin Testing (BET) Reagents Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.researchgate.net
Validation of the turbidimetric Limulus amebocyte lysate (LAL Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From minhvietco.com
Bioendo™ rFC Endotoxin Test Kit Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.bioendo.com
Professional Bioendo KT Endotoxin Test Kit Turbidimetric Assay Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.bioendo.com
Professional Factory wholesale Turbidimetric LAL Assay Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.accumedi.com
Endotoxin Assay Kit ( Clinical diagnosis for human, Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.accumedi.com
Endotoxin Assay Kit (Ampoule, with CSE and BET water, Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.slideserve.com
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.researchgate.net
(PDF) Optimization Sonication Time and Dilution Factor in Determining Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.accumedi.com
Endotoxin Assay Kit ( Clinical diagnosis for human, Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.slideserve.com
PPT Limulus Amebocyte Lysate (LAL) Test Methods PowerPoint Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From maxanim.com
Endotoxin Assay Kit (Special for dialysate, Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.bioendo.com
Professional Factory wholesale Turbidimetric LAL Assay Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.basciences.com
USP 85 Endotoxin Testing BA Sciences Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From dokumen.tips
(PDF) Turbidimetric Endotoxin Detection System DOKUMEN.TIPS Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.accumedi.com
Endotoxin Assay Kit (Ampoule, Special for dialysate, Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From de.bio-endo.com
Bioendo KTEndotoxinTestkit Turbidimetric Assay Kit KT0828 Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From vdocuments.mx
Turbidimetric Endotoxin Detection System [PDF Document] Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.lonzabioscience.com.au
PYROGENT™ 5000 Bulk Turbidimetric LAL Assay, 2,500 Tests Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide. Kinetic Turbidimetric Endotoxin Testing.
From www.semanticscholar.org
Figure 1 from Intralaboratory Validation of a Turbidimetric Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.accumedi.com
Endotoxin Assay Kit (Ampoule, Special for ACD solution, Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.bioendo.com
News turbidimetric endotoxin test assay is one of the Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant. Kinetic Turbidimetric Endotoxin Testing.
From www.sanichem.my
Whitepaper Bacterial Endotoxin Turbidimetric Interference Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From www.biophlox.com
Buy Bioendo KT Endotoxin Test Kit Turbidimetric Assay) get Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From ecsci.co.kr
PYROGENTTM5000 Turbidimetric LAL Assay, 200 Test Kit (주)에코셀 Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant. Kinetic Turbidimetric Endotoxin Testing.
From ko.bio-endo.com
Bioendo ™ KT Endotoxin Test Kit Turbidimetric Assay) 제조업체 및 공급 Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant. Kinetic Turbidimetric Endotoxin Testing.
From www.youtube.com
New FDA Expectations for Endotoxin Testing YouTube Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic lal assays are. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.
From dokumen.tips
(PDF) ENDOTOXIN DETECTION SYSTEMS · Flex tube reader or in an Kinetic Turbidimetric Endotoxin Testing The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.indiamart.com
Lonza Turbidimetric Bacterial Endotoxin Testing (BET) Reagents Kinetic Turbidimetric Endotoxin Testing Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. Both kinetic lal assays are. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant. Kinetic Turbidimetric Endotoxin Testing.
From www.bioendo.com
Professional Bioendo KT Endotoxin Test Kit Turbidimetric Assay Kinetic Turbidimetric Endotoxin Testing The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument such as the. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Both kinetic. Kinetic Turbidimetric Endotoxin Testing.
From www.talendotoxin.com
Bioendo™ KT Endotoxin Test Kit Turbidimetric Assay Kinetic Turbidimetric Endotoxin Testing Both kinetic lal assays are. The pyros kinetix ® flex instrument and pyros express 21 cfr part 11 compliant software provide a. Chromogenic and turbidimetric reagents yield quantitative endotoxin values and feature buffered formulations that provide robust testing with. The kinetic turbidimetric method or kta is perhaps the most commonly used method for endotoxin detection and requires a photometric instrument. Kinetic Turbidimetric Endotoxin Testing.