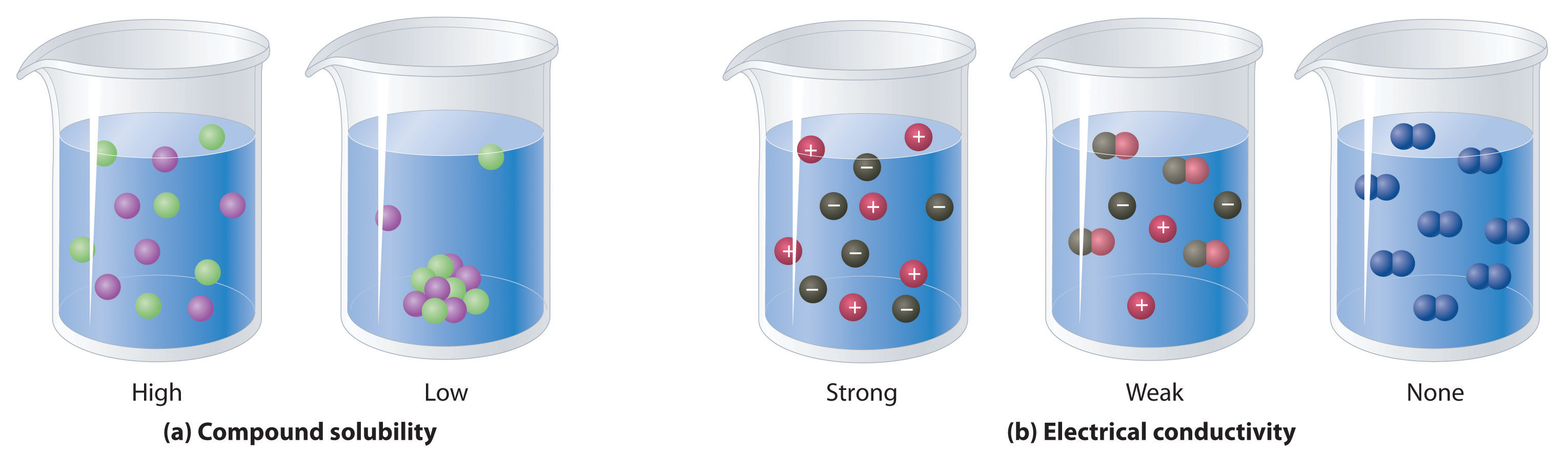
Lime Water Is An Aqueous Solution Of . Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Its chemical formula is ca(oh) 2. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. (i) lime water (aqueous calcium hydroxide): It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Lime water or milk of lime is the common name for saturated calcium hydroxide solution.
from saylordotorg.github.io
Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Its chemical formula is ca(oh) 2. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. (i) lime water (aqueous calcium hydroxide): It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications.
Aqueous Solutions
Lime Water Is An Aqueous Solution Of Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Its chemical formula is ca(oh) 2. (i) lime water (aqueous calcium hydroxide): Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Lime water or milk of lime is the common name for saturated calcium hydroxide solution.
From wou.edu
CH150 Chapter 7 Solutions Chemistry Lime Water Is An Aqueous Solution Of Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Its chemical formula is ca(oh) 2. (i) lime water (aqueous calcium hydroxide): Lime water or milk of lime is the common name for. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 Lime Water Is An Aqueous Solution Of (i) lime water (aqueous calcium hydroxide): Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g. Lime Water Is An Aqueous Solution Of.
From ajpoisson.blogspot.com
chemistry Aqueous solutions basics Lime Water Is An Aqueous Solution Of Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. (i) lime water (aqueous calcium hydroxide): Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Its chemical formula is ca(oh) 2. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. When. Lime Water Is An Aqueous Solution Of.
From www.doubtnut.com
Lime water is an aqueous solution of Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Lime water or milk of. Lime Water Is An Aqueous Solution Of.
From sitionroll.blogspot.com
Aqueous Solution Examples At Home sition Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. (i) lime water (aqueous calcium hydroxide): Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. It is prepared by stirring calcium hydroxide powder in pure water, and. Lime Water Is An Aqueous Solution Of.
From drowwater.com
Lime water Formula and Chemical Name? DrowWater Lime Water Is An Aqueous Solution Of Its chemical formula is ca(oh) 2. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Learn how to make it by mixing. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT Reactions in Aqueous Solutions PowerPoint Presentation, free Lime Water Is An Aqueous Solution Of (i) lime water (aqueous calcium hydroxide): Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. It is prepared by stirring calcium hydroxide. Lime Water Is An Aqueous Solution Of.
From saylordotorg.github.io
Aqueous Solutions Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Its chemical formula is ca(oh) 2. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. (i) lime water (aqueous calcium hydroxide): When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate. Lime Water Is An Aqueous Solution Of.
From www.chegg.com
Solved The following diagrams represent aqueous solutions of Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2.. Lime Water Is An Aqueous Solution Of.
From www.differencebetween.com
Difference Between Slaked Lime and Lime Water Compare the Difference Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. (i) lime water (aqueous calcium hydroxide): Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an. Lime Water Is An Aqueous Solution Of.
From www.studypool.com
SOLUTION To determine the concentration of a lime water solution Lime Water Is An Aqueous Solution Of Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT AQUEOUS SOLUTIONS PowerPoint Presentation, free download ID1197096 Lime Water Is An Aqueous Solution Of (i) lime water (aqueous calcium hydroxide): When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Limewater is a clear, colorless, aqueous solution of calcium. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT Chapter 4 Reactions in Aqueous Solutions PowerPoint Presentation Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. (i) lime water (aqueous calcium hydroxide): Its chemical formula is ca(oh) 2. When. Lime Water Is An Aqueous Solution Of.
From www.doubtnut.com
Lime water is an aqueous solution of Lime Water Is An Aqueous Solution Of Its chemical formula is ca(oh) 2. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Lime water or milk. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Lime Water Is An Aqueous Solution Of Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Its chemical formula is ca(oh) 2. Lime water. Lime Water Is An Aqueous Solution Of.
From www.chegg.com
Solved The diagrams below represent aqueous solutions that Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. When carbon dioxide reacts with water, it dissolves,. Lime Water Is An Aqueous Solution Of.
From users.highland.edu
Aqueous Solutions Lime Water Is An Aqueous Solution Of It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Its chemical formula is ca(oh) 2. (i) lime water (aqueous calcium hydroxide): Step by step video, text &. Lime Water Is An Aqueous Solution Of.
From www.slideshare.net
Chapter 8 Reactions in Aqueous Solution Lime Water Is An Aqueous Solution Of It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. (i) lime water (aqueous calcium hydroxide): When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to. Lime Water Is An Aqueous Solution Of.
From studylib.net
Reactions in Aqueous Solutions Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Its chemical formula is ca(oh) 2. Lime water or milk. Lime Water Is An Aqueous Solution Of.
From www.flinnsci.com
Flinn Chemicals, Limewater Solution Lime Water Is An Aqueous Solution Of Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Its chemical formula is ca(oh) 2. When carbon dioxide reacts with water, it dissolves, while some of it reacts with. Lime Water Is An Aqueous Solution Of.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, Lime Water Is An Aqueous Solution Of It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Learn how to. Lime Water Is An Aqueous Solution Of.
From www.youtube.com
How to make Lime Water Ca(OH)2 at home? Chemistry YouTube Lime Water Is An Aqueous Solution Of Lime water or milk of lime is the common name for saturated calcium hydroxide solution. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. (i) lime water (aqueous calcium hydroxide): Its chemical formula is ca(oh) 2. Learn how to make it by mixing calcium hydroxide and water, and see. Lime Water Is An Aqueous Solution Of.
From www.longevity-formulas.com
Lime Water — Longevity Formulas Lime Water Is An Aqueous Solution Of Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. It is prepared by stirring calcium hydroxide powder in pure. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT Ch 4. Chemical Quantities and Aqueous Reactions PowerPoint Lime Water Is An Aqueous Solution Of When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Step by step video, text & image solution for lime. Lime Water Is An Aqueous Solution Of.
From kolblabs.com
Preparation of lime water in ACIDS AND BASES Class 7 Science Experiment Lime Water Is An Aqueous Solution Of Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Its chemical formula is ca(oh) 2. Lime water or milk of lime is the common name for saturated calcium hydroxide solution.. Lime Water Is An Aqueous Solution Of.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Lime Water Is An Aqueous Solution Of Its chemical formula is ca(oh) 2. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. When carbon dioxide reacts with water, it. Lime Water Is An Aqueous Solution Of.
From www.carolina.com
Lime Water, Laboratory Grade Lime Water Is An Aqueous Solution Of (i) lime water (aqueous calcium hydroxide): Its chemical formula is ca(oh) 2. Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Lime water or milk of lime. Lime Water Is An Aqueous Solution Of.
From www.pinterest.com
Aqueous Solution Chemistry education, Solutions, Chemistry experiments Lime Water Is An Aqueous Solution Of Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Limewater is a clear, colorless, aqueous solution of calcium hydroxide. It is prepared. Lime Water Is An Aqueous Solution Of.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Lime Water Is An Aqueous Solution Of Lime water or milk of lime is the common name for saturated calcium hydroxide solution. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. (i) lime water (aqueous calcium hydroxide): Learn how to make it by mixing calcium hydroxide and water, and see its. Lime Water Is An Aqueous Solution Of.
From www.studypool.com
SOLUTION To determine the concentration of a lime water solution Lime Water Is An Aqueous Solution Of Step by step video, text & image solution for lime water is an aqueous solution of by chemistry experts to help you in doubts &. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. (i) lime water (aqueous calcium hydroxide): It is prepared by stirring calcium hydroxide powder in pure water, and filtering off. Lime Water Is An Aqueous Solution Of.
From www.doubtnut.com
Lime water is an aqueous solution of Lime Water Is An Aqueous Solution Of It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as. Lime Water Is An Aqueous Solution Of.
From www.slideserve.com
PPT Chapter 7 Properties of Solutions PowerPoint Presentation, free Lime Water Is An Aqueous Solution Of Its chemical formula is ca(oh) 2. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. It is prepared by stirring calcium hydroxide powder in pure water, and filtering off the excess undissolved ca (oh) 2. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. When carbon. Lime Water Is An Aqueous Solution Of.
From www.youtube.com
Action of Water on Quick Lime Combination Reaction YouTube Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. (i) lime water (aqueous calcium hydroxide): When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Learn how to make it by mixing calcium hydroxide and water, and see its properties and applications. It is. Lime Water Is An Aqueous Solution Of.
From byjus.com
What is the difference between lime water and slaked lime, though their Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. (i) lime water (aqueous calcium hydroxide): Ca(oh) 2 is only slightly soluble in water (0.16g ca(oh) 2 /100g water at 20°c) forming a basic solution called lime water. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known. Lime Water Is An Aqueous Solution Of.
From www.youtube.com
Passing Carbon dioxide Gas into Lime Water YouTube Lime Water Is An Aqueous Solution Of Limewater is a clear, colorless, aqueous solution of calcium hydroxide. Its chemical formula is ca(oh) 2. When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Lime water or milk of lime is the common name for saturated calcium hydroxide solution. Step by step video,. Lime Water Is An Aqueous Solution Of.