Magnesium Bicarbonate Reacts With Calcium Hydroxide . lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. reactions involving calcium carbonate.
from www.numerade.com
the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: reactions involving calcium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly.
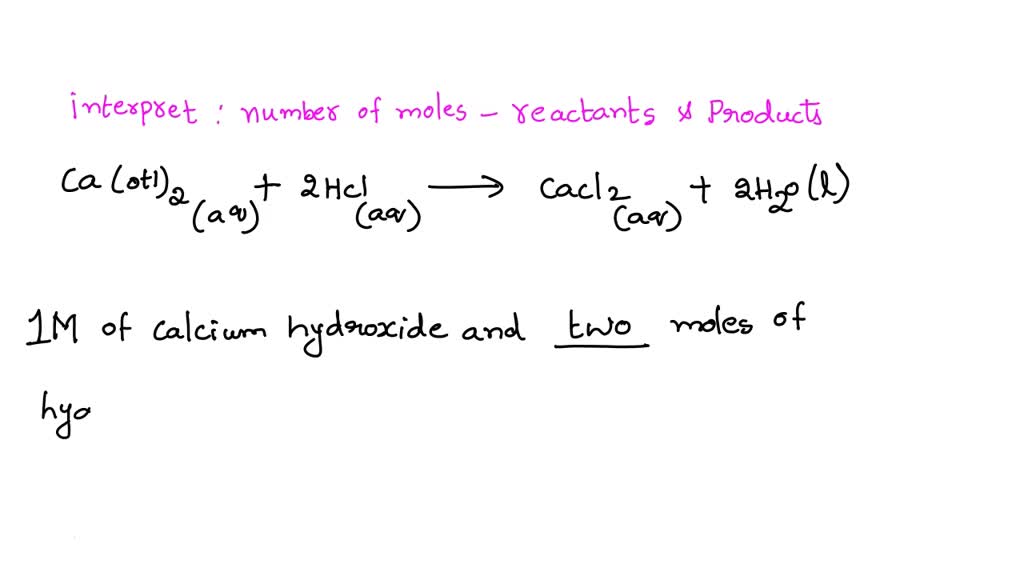
SOLVED The balanced chemical equation for the reaction between calcium
Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. reactions involving calcium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium.
From www.numerade.com
SOLVED Write chemical equations for the following reactions (1 Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. Then, the magnesium reacts with. reactions involving calcium carbonate. first,. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.studypool.com
SOLUTION Chemistry of sodium carbonate bicarbonate magnesium chloride Magnesium Bicarbonate Reacts With Calcium Hydroxide reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Then,. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.chemistrylearner.com
Calcium Bicarbonate Facts, Formula, Properties, Uses Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.numerade.com
SOLVED The isolation of magnesium from seawater can be summarized by Magnesium Bicarbonate Reacts With Calcium Hydroxide reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. first,. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From theheartysoul.com
Magnesium Bicarbonate Water Recipe for Headaches and Muscle Pain The Magnesium Bicarbonate Reacts With Calcium Hydroxide lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. the chemical reaction between magnesium bicarbonate. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.slideserve.com
PPT Reactions of calcium oxide, hydroxide, carbonate and bicarbonate Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. lime reacts with the calcium bicarbonate,. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between calcium Magnesium Bicarbonate Reacts With Calcium Hydroxide gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. reactions involving calcium carbonate. Then, the magnesium reacts with. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.youtube.com
Sodium Bicarbonate and Magnesium Sulphate YouTube Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From hyvida.com
Magnesium Bicarbonate Why is it so impactful? HyVIDA Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From slideplayer.com
Acid Base Equations. ppt download Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: lime. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.vrogue.co
Calcium Carbonate Reacts With Hydrobromic Acid To For vrogue.co Magnesium Bicarbonate Reacts With Calcium Hydroxide gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From slideplayer.com
Naming Binary Compounds ppt download Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. the. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.researchgate.net
Calcium, magnesium, bicarbonate and sulfate concentrations (mg/L Magnesium Bicarbonate Reacts With Calcium Hydroxide gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From slideplayer.com
Ch. 8 Chemical Reactions ppt download Magnesium Bicarbonate Reacts With Calcium Hydroxide the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. Then, the magnesium reacts with. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.tessshebaylo.com
Sodium Bicarbonate And Hydrochloric Acid Net Ionic Equation Tessshebaylo Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Then, the magnesium reacts with. first, magnesium bicarbonate reacts with. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.studypool.com
SOLUTION Chemistry of sodium carbonate bicarbonate magnesium chloride Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Then, the magnesium reacts with. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.slideshare.net
Chemical Reactions Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: gaseous. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Reacts With Calcium Hydroxide hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. the chemical reaction between magnesium bicarbonate. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution). Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.slideserve.com
PPT Reactions of calcium oxide, hydroxide, carbonate and bicarbonate Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. Then, the magnesium reacts with. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.youtube.com
Magnesium Bicarbonate Water (How I Make It) YouTube Magnesium Bicarbonate Reacts With Calcium Hydroxide hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. Then, the magnesium reacts with. reactions involving calcium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From fr.slideshare.net
Chemical Reactions Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. first, magnesium bicarbonate reacts. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.chegg.com
Solved Calcium and magnesium carbonates occur together in Magnesium Bicarbonate Reacts With Calcium Hydroxide hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: Then, the magnesium reacts with. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.slideserve.com
PPT Reactions of calcium oxide, hydroxide, carbonate and bicarbonate Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.tessshebaylo.com
Chemical Equation For Water And Calcium Tessshebaylo Magnesium Bicarbonate Reacts With Calcium Hydroxide reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. first, magnesium bicarbonate reacts with lime. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.vedantu.com
Magnesium Bicarbonate Learn Important Terms and Concepts Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. lime reacts with the calcium bicarbonate, making calcium carbonate, which. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.pinterest.com
How to make a potent Magnesium Bicarbonate solution Magnesium water Magnesium Bicarbonate Reacts With Calcium Hydroxide the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. Then, the magnesium reacts with. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From scihub.world
Calcium Carbonate Nitric Acid Balanced Equation Magnesium Bicarbonate Reacts With Calcium Hydroxide hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. reactions involving calcium carbonate. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. first, magnesium bicarbonate reacts with lime and produces calcium carbonate. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.researchgate.net
(PDF) The Effect of Magnesium Hydroxide Addition on the Extinguishing Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: reactions involving calcium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From exoennldl.blob.core.windows.net
Bicarbonate Magnesium Equation at David Stevenson blog Magnesium Bicarbonate Reacts With Calcium Hydroxide lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. hydrochloric. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.slideserve.com
PPT Reactions of calcium oxide, hydroxide, carbonate and bicarbonate Magnesium Bicarbonate Reacts With Calcium Hydroxide hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. reactions involving calcium carbonate. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Of The... Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. the chemical reaction between magnesium bicarbonate and calcium hydroxide forms magnesium hydroxide and calcium. lime reacts with the calcium bicarbonate, making calcium carbonate, which settles very quickly. reactions involving calcium carbonate. hydrochloric. Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From www.toppr.com
100mL of a water sample contains 0.81g of calcium bicarbonate and 0.73 Magnesium Bicarbonate Reacts With Calcium Hydroxide Then, the magnesium reacts with. first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: . Magnesium Bicarbonate Reacts With Calcium Hydroxide.
From exoennldl.blob.core.windows.net
Bicarbonate Magnesium Equation at David Stevenson blog Magnesium Bicarbonate Reacts With Calcium Hydroxide first, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate. gaseous butane, c 4 h 10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water: reactions involving calcium carbonate. . Magnesium Bicarbonate Reacts With Calcium Hydroxide.