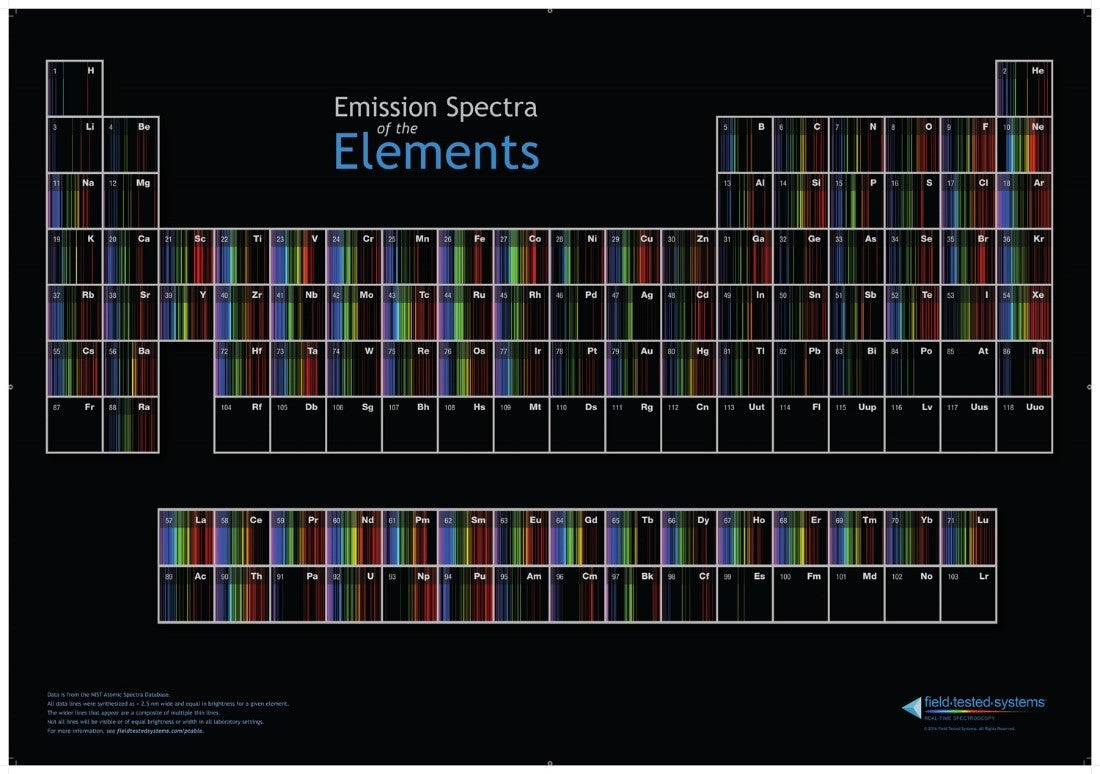
Emission Spectra Different Elements . If their energy is increased, then they can jump to a. Electrons move rapidly around the nucleus in energy shells. The emission spectra of various atoms. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The actual wavelengths of the lines are predictably. Absorption spectra are lit with dark bands;
from uwaterloo.ca
The emission spectra of various atoms. Absorption spectra are lit with dark bands; If their energy is increased, then they can jump to a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Every atomic element has a unique absorption and emission spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a.
Periodic Table of emission spectra Chem 13 News Magazine University
Emission Spectra Different Elements The actual wavelengths of the lines are predictably. Absorption spectra are lit with dark bands; If their energy is increased, then they can jump to a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Every atomic element has a unique absorption and emission spectrum. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectra of various atoms. The actual wavelengths of the lines are predictably. Electrons move rapidly around the nucleus in energy shells.
From ar.inspiredpencil.com
Spectrum Noble Gases Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Absorption spectra are lit with dark bands; The actual wavelengths of the lines are predictably. The emission spectra of various atoms. Every atomic element has a unique absorption and emission spectrum. If their energy is increased, then. Emission Spectra Different Elements.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Different Elements Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Absorption spectra are lit with dark bands; The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. Every atomic element has a unique absorption and emission spectrum. The emission spectra of various atoms. Electrons move. Emission Spectra Different Elements.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Electrons move rapidly around the nucleus in energy shells. The actual wavelengths of the lines are predictably. Although objects at high temperature. Emission Spectra Different Elements.
From umop.net
Visible Spectra of the Elements Emission Spectra Different Elements Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Electrons move. Emission Spectra Different Elements.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. If their energy is increased, then they can jump to a. Every atomic element has. Emission Spectra Different Elements.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances. If you Emission Spectra Different Elements The actual wavelengths of the lines are predictably. Every atomic element has a unique absorption and emission spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Different Elements.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Every atomic element has a unique absorption and emission spectrum. The actual wavelengths of the lines are predictably. Absorption spectra are lit. Emission Spectra Different Elements.
From www.savemyexams.com
Flame Emission Spectroscopy AQA GCSE Chemistry Revision Notes 2018 Emission Spectra Different Elements Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Every atomic element has a unique absorption and emission spectrum. The spectroscope separates the light. Emission Spectra Different Elements.
From umop.net
Visible Spectra of the Elements Emission Spectra Different Elements If their energy is increased, then they can jump to a. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The actual wavelengths of the lines are predictably. Electrons move rapidly around the nucleus in energy shells. Absorption spectra are lit with dark bands; The emission spectra of various atoms. Every atomic element has a unique. Emission Spectra Different Elements.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Emission Spectra Different Elements Every atomic element has a unique absorption and emission spectrum. The emission spectra of various atoms. Electrons move rapidly around the nucleus in energy shells. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The actual wavelengths of the lines are predictably. If their energy is increased, then they can jump to a. The emission spectrum. Emission Spectra Different Elements.
From mrsmorrittscience.weebly.com
Emission Spectra of the Elements Arranged on the Periodic Table Mrs Emission Spectra Different Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. If their energy is increased, then they can jump to a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Although objects. Emission Spectra Different Elements.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Different Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Electrons move rapidly around the nucleus in energy shells. The emission spectra of various atoms. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are. Emission Spectra Different Elements.
From www.alamy.com
Diagram showing the absorption and emission spectra of the elements Emission Spectra Different Elements Absorption spectra are lit with dark bands; The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Every atomic element has a unique absorption and emission spectrum. The actual wavelengths of the lines are predictably. If their energy is increased, then they can jump to a. Although. Emission Spectra Different Elements.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Different Elements The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Every atomic element has a unique absorption and emission spectrum. Electrons move rapidly around the nucleus in energy shells. The spectroscope separates the light of varying wavelengths and we observe. Emission Spectra Different Elements.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectra Different Elements Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands; The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Emission Spectra Different Elements.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Different Elements If their energy is increased, then they can jump to a. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The actual wavelengths of the lines are predictably. Electrons move rapidly around the nucleus in. Emission Spectra Different Elements.
From users.highland.edu
Atomic Spectra and Models of the Atom Emission Spectra Different Elements If their energy is increased, then they can jump to a. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Absorption spectra are lit with dark bands; The actual wavelengths of the. Emission Spectra Different Elements.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Different Elements The emission spectra of various atoms. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Electrons move rapidly around the nucleus in energy shells. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The spectroscope separates the light of varying wavelengths. Emission Spectra Different Elements.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectra Different Elements Every atomic element has a unique absorption and emission spectrum. Absorption spectra are lit with dark bands; The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Emission Spectra Different Elements.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Different Elements The emission spectra of various atoms. If their energy is increased, then they can jump to a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Absorption spectra are lit with dark bands;. Emission Spectra Different Elements.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Emission Spectra Different Elements Electrons move rapidly around the nucleus in energy shells. The actual wavelengths of the lines are predictably. Absorption spectra are lit with dark bands; If their energy is increased, then they can jump to a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectra of various atoms. Although objects at high temperature. Emission Spectra Different Elements.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra Different Elements Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The actual wavelengths of the lines are predictably. Electrons move rapidly around the nucleus in energy shells. Absorption spectra are lit with dark bands; If their energy is increased, then they can jump to a. The emission spectra of various atoms. Every atomic element has a unique. Emission Spectra Different Elements.
From uwaterloo.ca
Periodic Table of emission spectra Chem 13 News Magazine University Emission Spectra Different Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Every atomic element has a unique absorption and emission spectrum. The emission spectra of various atoms. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The spectroscope separates the light of varying. Emission Spectra Different Elements.
From www.universetoday.com
Spectroscopy The Key to Humanity's Future in Space Universe Today Emission Spectra Different Elements The actual wavelengths of the lines are predictably. Absorption spectra are lit with dark bands; Electrons move rapidly around the nucleus in energy shells. Every atomic element has a unique absorption and emission spectrum. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element is the. Emission Spectra Different Elements.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Emission Spectra Different Elements Absorption spectra are lit with dark bands; Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Electrons move rapidly around the nucleus in energy shells. Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of. Emission Spectra Different Elements.
From www.thoughtco.com
Balmer Series Definition in Science Emission Spectra Different Elements The emission spectra of various atoms. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Absorption spectra are lit with dark bands; Electrons move rapidly around the nucleus in energy shells. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. If their energy is increased, then they can jump to a.. Emission Spectra Different Elements.
From www.reddit.com
Periodic table of each elements emission spectrum with regular periodic Emission Spectra Different Elements The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Every atomic element has a unique absorption and emission spectrum. Electrons move rapidly around the nucleus in energy shells. If their energy is increased, then they can jump. Emission Spectra Different Elements.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Different Elements Electrons move rapidly around the nucleus in energy shells. If their energy is increased, then they can jump to a. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Absorption spectra are lit with dark bands; The emission spectra of various atoms.. Emission Spectra Different Elements.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Emission Spectra Different Elements The emission spectra of various atoms. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Absorption spectra are lit with dark bands; Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. Emission Spectra Different Elements.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The emission spectra of various atoms. Electrons move rapidly around the nucleus in energy shells. Absorption spectra are lit with dark bands; The emission spectrum (or line spectrum) of a chemical element is. Emission Spectra Different Elements.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectra Different Elements If their energy is increased, then they can jump to a. Every atomic element has a unique absorption and emission spectrum. The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The spectroscope separates the light of varying. Emission Spectra Different Elements.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Different Elements If their energy is increased, then they can jump to a. Every atomic element has a unique absorption and emission spectrum. The actual wavelengths of the lines are predictably. The emission spectra of various atoms. Absorption spectra are lit with dark bands; Electrons move rapidly around the nucleus in energy shells. The emission spectrum (or line spectrum) of a chemical. Emission Spectra Different Elements.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Emission Spectra Different Elements Electrons move rapidly around the nucleus in energy shells. Every atomic element has a unique absorption and emission spectrum. The actual wavelengths of the lines are predictably. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. Emission Spectra Different Elements.
From studylib.net
Every element has its own characteristic spectral emission Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. Absorption spectra are lit with dark bands; Every atomic element has a unique absorption and emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Emission Spectra Different Elements.
From www.astronoo.com
Spectroscopy — Astronoo Emission Spectra Different Elements The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Every atomic element has a unique absorption and emission spectrum. If their energy is increased, then they can jump to a. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a. Absorption spectra are lit with dark bands; Electrons move rapidly around the. Emission Spectra Different Elements.