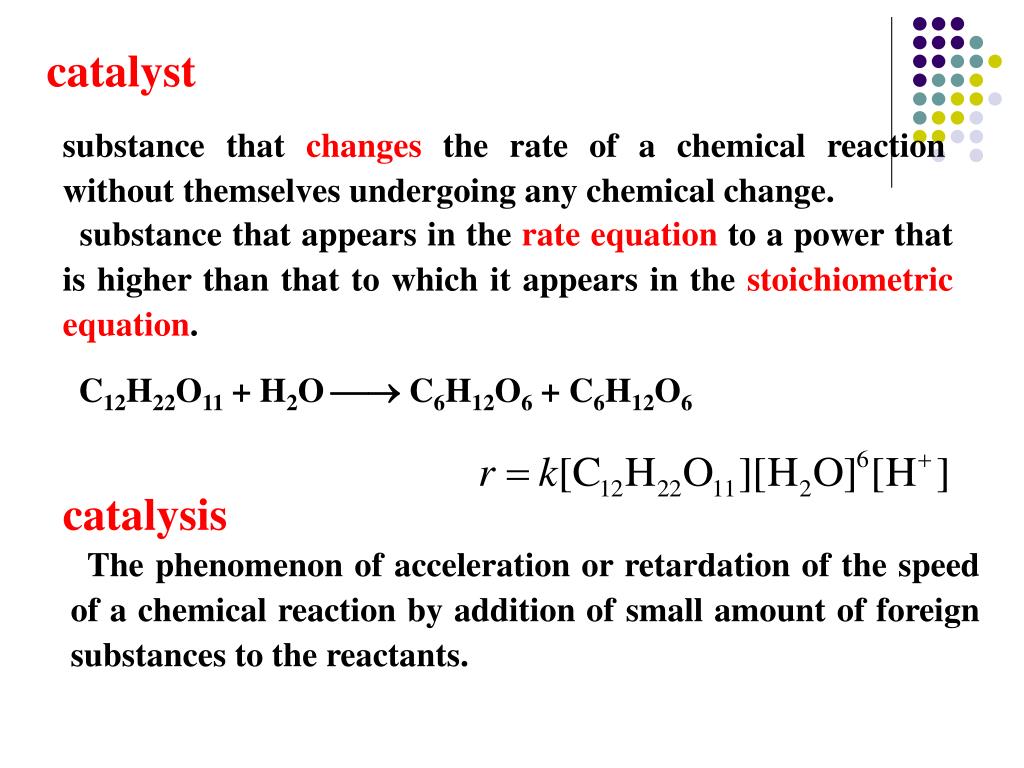
Catalyst Examples Substances . a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. list examples of catalysis in natural and industrial processes. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. The catalyst is not used up or chemically changed during the reaction. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. a catalyst is a substance that speeds up a chemical reaction. These substances help speed up chemical reactions so that they proceed efficiently. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — what are catalysts? Among the factors affecting chemical reaction rates.
from exobghamq.blob.core.windows.net
— potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. These substances help speed up chemical reactions so that they proceed efficiently. list examples of catalysis in natural and industrial processes. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. — what are catalysts? A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. The catalyst is not used up or chemically changed during the reaction. Among the factors affecting chemical reaction rates. a catalyst is a substance that speeds up a chemical reaction.
Catalysts Chemical Equation at Joy Perry blog
Catalyst Examples Substances — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. — what are catalysts? a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. a catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. These substances help speed up chemical reactions so that they proceed efficiently. list examples of catalysis in natural and industrial processes. The catalyst is not used up or chemically changed during the reaction. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Among the factors affecting chemical reaction rates.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog Catalyst Examples Substances A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. a catalyst is a substance that speeds up a chemical reaction. The catalyst. Catalyst Examples Substances.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalyst Examples Substances Among the factors affecting chemical reaction rates. — what are catalysts? A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. a catalyst is a substance that speeds up a chemical reaction. a catalyst is a substance that increases the rate of a chemical reaction by. Catalyst Examples Substances.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalyst Examples Substances a catalyst is a substance that speeds up a chemical reaction. — what are catalysts? Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. list examples of catalysis in natural and industrial processes. These substances help speed up chemical reactions so. Catalyst Examples Substances.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalyst Examples Substances These substances help speed up chemical reactions so that they proceed efficiently. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. a catalyst is a substance that speeds up a chemical reaction. list examples of catalysis in natural and industrial processes. Among the factors affecting chemical. Catalyst Examples Substances.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Examples Substances These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that speeds up a chemical reaction. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. list examples of catalysis in natural and industrial processes. Among the. Catalyst Examples Substances.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalyst Examples Substances — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide. Catalyst Examples Substances.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog Catalyst Examples Substances a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. Among the factors affecting chemical reaction rates. list examples of catalysis in natural and industrial processes. a catalyst is a substance that speeds up a chemical reaction. — catalyst, in chemistry, any substance. Catalyst Examples Substances.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download ID5923586 Catalyst Examples Substances These substances help speed up chemical reactions so that they proceed efficiently. list examples of catalysis in natural and industrial processes. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. a catalyst is a substance that increases the rate of a chemical reaction by lowering the. Catalyst Examples Substances.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID6634764 Catalyst Examples Substances The catalyst is not used up or chemically changed during the reaction. Among the factors affecting chemical reaction rates. — what are catalysts? a catalyst is a substance that speeds up a chemical reaction. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. list examples of catalysis in. Catalyst Examples Substances.
From www.slideshare.net
Biology 2.4 Catalyst Examples Substances — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that increases the rate of a. Catalyst Examples Substances.
From examples.yourdictionary.com
Examples of Catalysts YourDictionary Catalyst Examples Substances — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — what are catalysts? list examples of catalysis in natural and industrial processes. The catalyst is not used up or chemically changed during the reaction. a catalyst is a substance that speeds up a chemical reaction. These substances help. Catalyst Examples Substances.
From www.slideshare.net
Catalyst Catalyst Examples Substances — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. These substances help speed up chemical reactions so that they proceed efficiently. Among the factors affecting chemical reaction. Catalyst Examples Substances.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID4752647 Catalyst Examples Substances — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. These substances help speed up chemical reactions so that they proceed efficiently. The catalyst is not used up or chemically changed during the reaction. Among the factors affecting chemical reaction rates. list examples of catalysis in natural and industrial processes. . Catalyst Examples Substances.
From slideplayer.com
Unit 1 Chemistry (P ) Patterns and Compounds ppt download Catalyst Examples Substances — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. a catalyst is a substance that speeds up a chemical reaction. list examples of catalysis in natural and industrial. Catalyst Examples Substances.
From www.slideserve.com
PPT Catalysts and Rate of Reaction PowerPoint Presentation, free download ID2077937 Catalyst Examples Substances a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. The catalyst is not used up or chemically changed during the reaction. a catalyst is a substance. Catalyst Examples Substances.
From www.slideserve.com
PPT Transition metals catalysts PowerPoint Presentation, free download ID2121239 Catalyst Examples Substances — what are catalysts? a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds up a chemical reaction, or lowers. Catalyst Examples Substances.
From exonprxge.blob.core.windows.net
Catalyst Examples In Life at Anthony Snyder blog Catalyst Examples Substances — what are catalysts? The catalyst is not used up or chemically changed during the reaction. These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that speeds up a chemical reaction. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. . Catalyst Examples Substances.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Presentation ID3058051 Catalyst Examples Substances a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. These substances help speed up chemical reactions so that they proceed efficiently. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and. Catalyst Examples Substances.
From slideplayer.com
Catalysts May SCH4U1. ppt download Catalyst Examples Substances Among the factors affecting chemical reaction rates. The catalyst is not used up or chemically changed during the reaction. These substances help speed up chemical reactions so that they proceed efficiently. list examples of catalysis in natural and industrial processes. a catalyst is a substance that speeds up a chemical reaction. a catalyst is a substance that. Catalyst Examples Substances.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID2075869 Catalyst Examples Substances a catalyst is a substance that speeds up a chemical reaction. These substances help speed up chemical reactions so that they proceed efficiently. — what are catalysts? — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. a catalyst is a substance that increases the rate of a chemical. Catalyst Examples Substances.
From www.slideshare.net
Catalysis Catalyst Examples Substances list examples of catalysis in natural and industrial processes. Among the factors affecting chemical reaction rates. — what are catalysts? a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. a catalyst is a substance that speeds up a chemical reaction. These substances. Catalyst Examples Substances.
From exowzdqkr.blob.core.windows.net
Catalyst Biology Image at Anna Schofield blog Catalyst Examples Substances The catalyst is not used up or chemically changed during the reaction. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. — catalyst, in chemistry, any substance that increases. Catalyst Examples Substances.
From www.slideshare.net
Catalysis Catalyst Examples Substances — what are catalysts? list examples of catalysis in natural and industrial processes. These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. a catalyst is a substance that speeds up. Catalyst Examples Substances.
From www.tes.com
New AQA A2 Organic chemistry Transition metals catalysts Teaching Resources Catalyst Examples Substances — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. These substances help speed up chemical reactions so that they proceed efficiently. The catalyst is not used up or chemically changed. Catalyst Examples Substances.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalyst Examples Substances These substances help speed up chemical reactions so that they proceed efficiently. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up. Catalyst Examples Substances.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions, Chemistry education Catalyst Examples Substances A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. The catalyst is. Catalyst Examples Substances.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalyst Examples Substances list examples of catalysis in natural and industrial processes. — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. a catalyst is a substance that. Catalyst Examples Substances.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Examples Substances — what are catalysts? A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that speeds up a chemical reaction. — potassium permanganate acts as a catalyst for the. Catalyst Examples Substances.
From exoluszth.blob.core.windows.net
Catalyst Examples Pictures at Mandy Simpkins blog Catalyst Examples Substances a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. These substances help speed up chemical reactions so that they proceed efficiently. Among the factors affecting chemical reaction rates. The catalyst is not used up or chemically changed during the reaction. list examples of catalysis. Catalyst Examples Substances.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Examples Substances list examples of catalysis in natural and industrial processes. Among the factors affecting chemical reaction rates. These substances help speed up chemical reactions so that they proceed efficiently. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. A catalyst is a substance that speeds. Catalyst Examples Substances.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID2087208 Catalyst Examples Substances a catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed during the reaction. a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. A catalyst is a substance that speeds up a chemical reaction, or. Catalyst Examples Substances.
From www.slideserve.com
PPT Catalysts and Rate of Reaction PowerPoint Presentation, free download ID2077937 Catalyst Examples Substances a catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed during the reaction. Among the factors affecting chemical reaction rates. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. — what are catalysts? a catalyst is a substance. Catalyst Examples Substances.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Examples Substances list examples of catalysis in natural and industrial processes. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one,. The catalyst is not used up or chemically changed during the reaction. Among the factors affecting chemical reaction rates. — catalyst, in chemistry, any substance that increases the. Catalyst Examples Substances.
From exoluszth.blob.core.windows.net
Catalyst Examples Pictures at Mandy Simpkins blog Catalyst Examples Substances a catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. — what are catalysts? A catalyst is a substance that speeds up a chemical reaction, or lowers. Catalyst Examples Substances.
From www.slideserve.com
PPT IB Chemistry PowerPoint Presentation, free download ID2738432 Catalyst Examples Substances These substances help speed up chemical reactions so that they proceed efficiently. — potassium permanganate acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. — what are catalysts? — catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a substance that speeds. Catalyst Examples Substances.