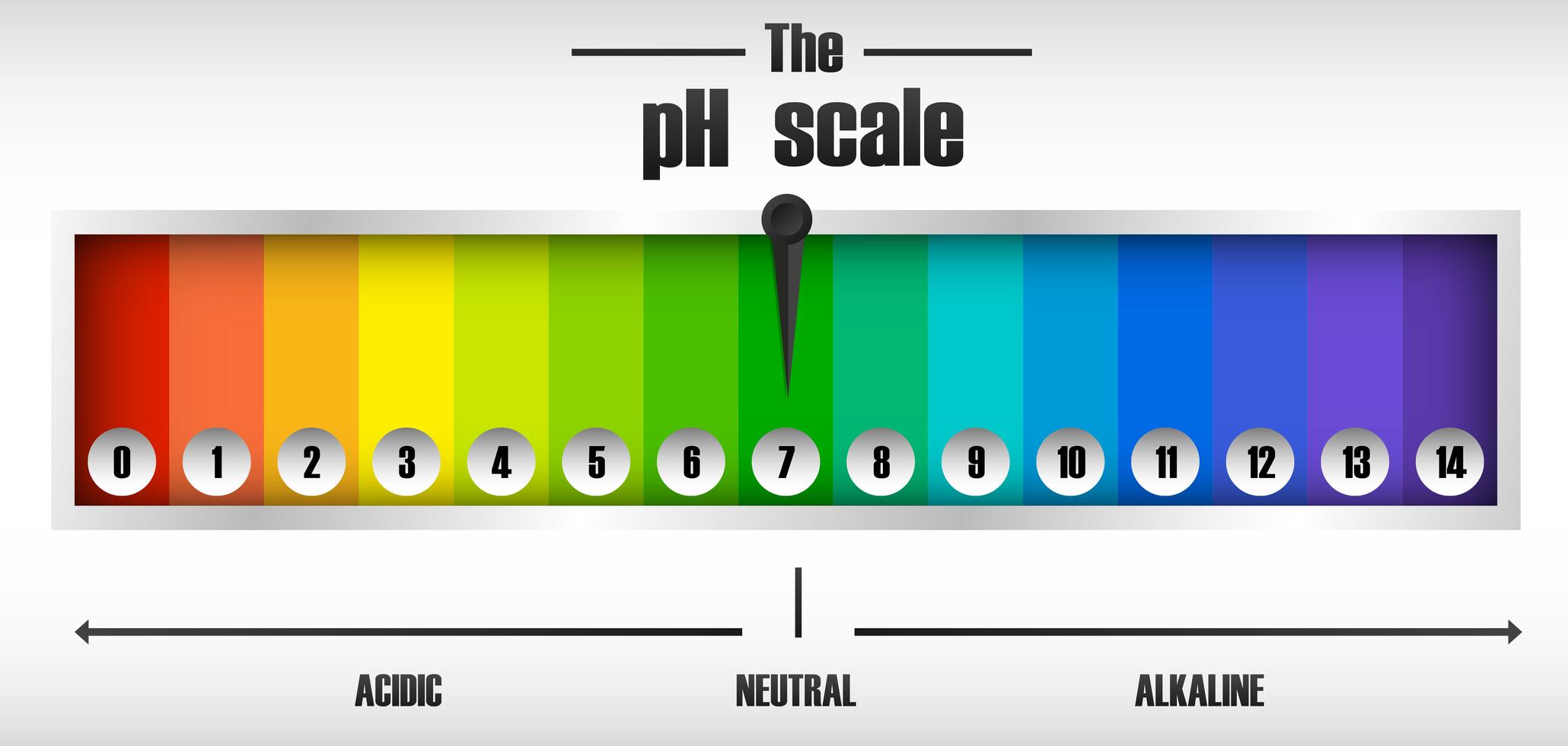
Ph Indicator Simple Definition . They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Normally, the indicator causes the. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. indicators are substances whose solutions change color due to changes in ph. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing.
from es.vecteezy.com
Normally, the indicator causes the. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing.
El diagrama de la escala de ph 589313 Vector en Vecteezy
Ph Indicator Simple Definition indicators are substances whose solutions change color due to changes in ph. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. Normally, the indicator causes the. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock Ph Indicator Simple Definition Normally, the indicator causes the. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. A ph value is determined. Ph Indicator Simple Definition.
From www.expii.com
What Is pH? — Definition & Overview Expii Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. ph indicators are weak acids that. Ph Indicator Simple Definition.
From www.shutterstock.com
Universal Indicator Ph Color Chart Scientific Stock Vector (Royalty Ph Indicator Simple Definition indicators are substances whose solutions change color due to changes in ph. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Normally, the indicator causes the. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h +. Ph Indicator Simple Definition.
From www.dreamstime.com
PH Scale. Universal Indicator PH Stock Vector Illustration of healthy Ph Indicator Simple Definition the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions. Ph Indicator Simple Definition.
From www.slideserve.com
PPT Solutions and pH PowerPoint Presentation, free download ID5498133 Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). Normally, the indicator causes the. They are usually weak acids or. Ph Indicator Simple Definition.
From www.homeworkjoy.com
The pH Scale with Examples Common Indicators Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. indicators are substances whose. Ph Indicator Simple Definition.
From dxoufetkd.blob.core.windows.net
Indicators Of Bases at Sara Bowling blog Ph Indicator Simple Definition They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph. Ph Indicator Simple Definition.
From www.waca.msf.org
What is PH Definition, Ph Ph Indicator Simple Definition Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. A ph value is determined from the negative logarithm of this concentration and is used to indicate the. Ph Indicator Simple Definition.
From www.worksheetsplanet.com
What is Ph Scale Definition of Ph Scale Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). Normally, the indicator causes the. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. They are usually weak acids or bases, but their. Ph Indicator Simple Definition.
From www.thoughtco.com
pH Definition and Equation in Chemistry Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. Normally, the indicator causes the. indicators are substances whose solutions. Ph Indicator Simple Definition.
From www.thoughtco.com
What Is a pH Indicator? Definition and Examples Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different. Ph Indicator Simple Definition.
From www.vectorstock.com
Ph scale universal indicator ph color chart Vector Image Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. A ph value is determined from the negative logarithm of this concentration and is used to. Ph Indicator Simple Definition.
From chemstuff.co.uk
341259_Bio0217pH_Scale.png Chemstuff Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. They are usually weak acids. Ph Indicator Simple Definition.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). the ph scale measures how strongly acidic or alkaline a. Ph Indicator Simple Definition.
From www.dreamstime.com
The Ph Scale Universal Indicator Ph Color Chart Diagram. Vector Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. the ph indicator is a chemical. Ph Indicator Simple Definition.
From microbenotes.com
pH Meter Principle, Parts, Procedure, Types, Uses, Examples Ph Indicator Simple Definition the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). Normally, the indicator causes the. They are usually weak acids or bases, but their conjugate base. Ph Indicator Simple Definition.
From www.alamy.com
The Ph scale universal Indicator ph Color Chart diagram. Vector Ph Indicator Simple Definition Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a. Ph Indicator Simple Definition.
From es.vecteezy.com
El diagrama de la escala de ph 589313 Vector en Vecteezy Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Normally, the indicator causes the. the. Ph Indicator Simple Definition.
From www.alamy.com
scale of ph value for acid and alkaline solutions, infographic acid Ph Indicator Simple Definition indicators are substances whose solutions change color due to changes in ph. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. Normally, the indicator causes the. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or. Ph Indicator Simple Definition.
From www.flinnsci.com
pH Indicator Chart Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. indicators are substances whose solutions change color due to changes in ph. A ph value. Ph Indicator Simple Definition.
From www.dreamstime.com
The PH Scale Universal Indicator PH Color Chart Diagram Stock Vector Ph Indicator Simple Definition Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. the ph scale measures how strongly acidic or alkaline a solution is using a set. Ph Indicator Simple Definition.
From www.dreamstime.com
PH Scale Diagram with Corresponding Acidic or Alcaline Values Ph Indicator Simple Definition Normally, the indicator causes the. indicators are substances whose solutions change color due to changes in ph. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. A ph value is determined from the negative logarithm of this concentration and is used to indicate the. Ph Indicator Simple Definition.
From www.science-sparks.com
What is the pH Scale? Ph Indicator Simple Definition the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. Normally, the indicator causes the. ph. Ph Indicator Simple Definition.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). the ph scale measures how strongly acidic or alkaline a solution is using. Ph Indicator Simple Definition.
From mavink.com
Types Of Ph Indicators Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. indicators are substances whose solutions change color due to changes in ph. A ph value. Ph Indicator Simple Definition.
From www.thoughtco.com
What Is a pH Indicator? Definition and Examples Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. They are usually weak acids. Ph Indicator Simple Definition.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock Ph Indicator Simple Definition indicators are substances whose solutions change color due to changes in ph. ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0. Ph Indicator Simple Definition.
From www.youtube.com
A Simple Guide to the pH Scale and Indicators YouTube Ph Indicator Simple Definition They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. the ph indicator is a chemical detector for hydronium ions (h. Ph Indicator Simple Definition.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. Normally, the indicator causes the. the. Ph Indicator Simple Definition.
From www.alamy.com
The pH scale Universal Indicator pH Color Chart diagram acidic Stock Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. Normally, the indicator causes the. the ph scale measures how strongly acidic or alkaline a solution is using a set of values from ph 0 to ph 14. indicators are substances whose. Ph Indicator Simple Definition.
From www.dreamstime.com
PH Scale Diagram with Corresponding Acidic or Alcaline Values Ph Indicator Simple Definition the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. ph indicators are weak acids that exist as natural dyes and indicate the concentration of. Ph Indicator Simple Definition.
From www.chemswot.com
Definition of pH scale in Chemistry Ph Indicator Simple Definition They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. indicators are substances whose solutions change. Ph Indicator Simple Definition.
From www.vectorstock.com
Ph scale universal indicator ph color chart Vector Image Ph Indicator Simple Definition ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\(h_3o^+\)) ions in a solution via color change. indicators are substances whose solutions change color due to changes in ph. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or. Ph Indicator Simple Definition.
From study.com
pH Determination Overview & Methods Lesson Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Normally, the indicator causes the. the. Ph Indicator Simple Definition.
From www.vedantu.com
Defining pH Indicator and Understanding its Importance in Life Ph Indicator Simple Definition A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. the ph indicator is a chemical detector for hydronium ions (h 3 o +) or hydrogen ions (h +). ph indicators are weak acids that exist as natural dyes. Ph Indicator Simple Definition.