Aluminum Oxide Balanced Equation . To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It shows the reactants (substances that start a reaction) and products (substances. Explain the roles of subscripts and coefficients in chemical equations. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the unbalanced equation. The balanced equation will appear. A chemical equation represents a chemical reaction. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? In order to balance al + o2 = al2o3 you'll need to watch out for two things. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Aluminum oxide = aluminium + dioxygen.
from www.doubtnut.com
In order to balance al + o2 = al2o3 you'll need to watch out for two things. A chemical equation represents a chemical reaction. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Aluminum oxide = aluminium + dioxygen. It shows the reactants (substances that start a reaction) and products (substances. Explain the roles of subscripts and coefficients in chemical equations. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Balance a chemical equation when given the unbalanced equation. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate?
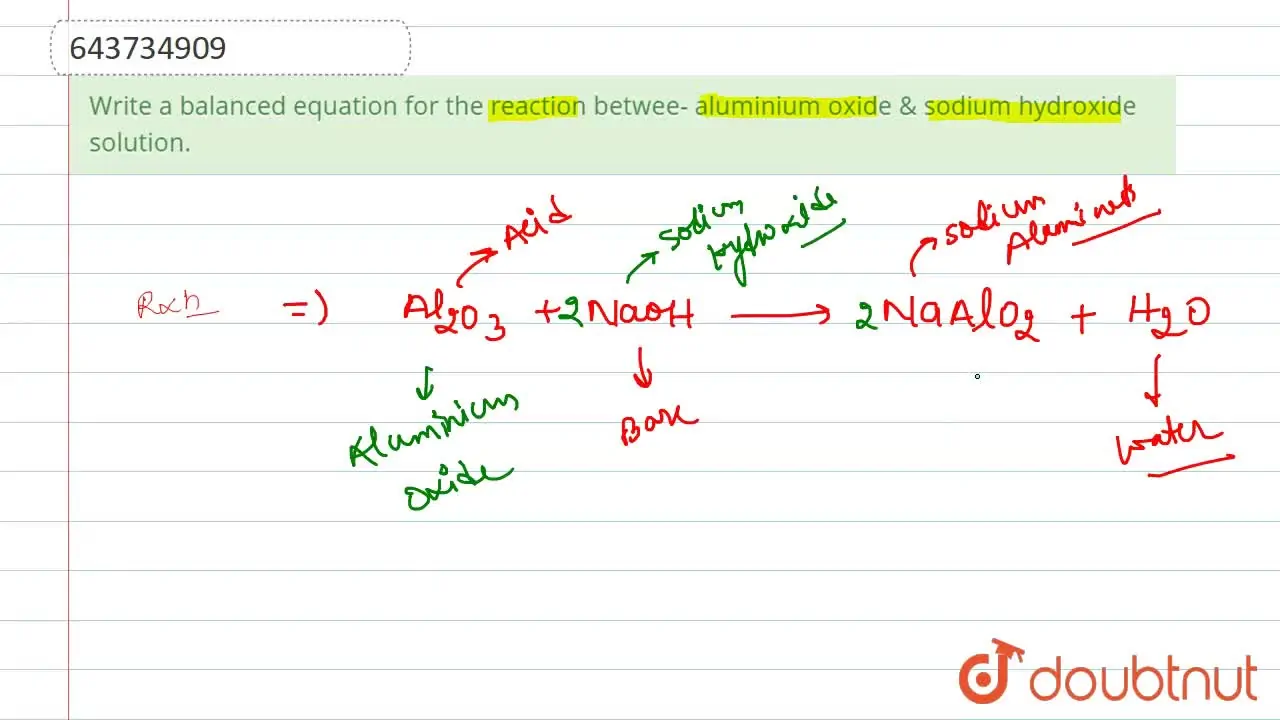
Write a balanced equation for the reaction betwee aluminium oxide & s
Aluminum Oxide Balanced Equation How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Balance a chemical equation when given the unbalanced equation. In order to balance al + o2 = al2o3 you'll need to watch out for two things. Aluminum oxide = aluminium + dioxygen. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction.
From www.numerade.com
A chemist burns 160.0 g of Al in excess air to produce aluminum oxide Aluminum Oxide Balanced Equation The balanced equation will appear. Aluminum oxide = aluminium + dioxygen. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Explain the roles of subscripts and coefficients in chemical equations. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. It. Aluminum Oxide Balanced Equation.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas Aluminum Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. Aluminum oxide = aluminium + dioxygen. Explain the roles of subscripts and coefficients in chemical equations. In order to balance al + o2 = al2o3 you'll need to watch out for two things. How do you write the. Aluminum Oxide Balanced Equation.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. The balanced equation will appear. A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances. Explain the roles of subscripts and coefficients in chemical equations. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. How do. Aluminum Oxide Balanced Equation.
From www.youtube.com
How to Balance Al + O2 = Al2O3 YouTube Aluminum Oxide Balanced Equation How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction. Aluminum oxide =. Aluminum Oxide Balanced Equation.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free Aluminum Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the unbalanced equation. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? It shows the reactants (substances that start a reaction) and products (substances. In. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Aluminum metal reacts with oxygen gas to produce aluminum oxide Aluminum Oxide Balanced Equation A chemical equation represents a chemical reaction. In order to balance al + o2 = al2o3 you'll need to watch out for two things. Aluminum oxide = aluminium + dioxygen. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. How do you write the balanced molecular and net ionic equations for. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVEDAluminum oxidizes to form aluminum oxide. a. Write the balanced Aluminum Oxide Balanced Equation Aluminum oxide = aluminium + dioxygen. Explain the roles of subscripts and coefficients in chemical equations. In order to balance al + o2 = al2o3 you'll need to watch out for two things. A chemical equation represents a chemical reaction. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED a chemist burns 16.0 g of al in excess air to produce aluminum Aluminum Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. It shows the reactants (substances that start. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide Aluminum Oxide Balanced Equation It shows the reactants (substances that start a reaction) and products (substances. In order to balance al + o2 = al2o3 you'll need to watch out for two things. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. How do you write. Aluminum Oxide Balanced Equation.
From byjus.com
59 Write the reactions at cathode and anode during electrolytic Aluminum Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Aluminum oxide = aluminium + dioxygen. The balanced equation will appear. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Explain the. Aluminum Oxide Balanced Equation.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Aluminum Oxide Balanced Equation The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. A chemical equation represents a chemical reaction. Aluminum oxide = aluminium + dioxygen. To balance a chemical equation,. Aluminum Oxide Balanced Equation.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Balanced Equation Aluminum oxide = aluminium + dioxygen. Balance a chemical equation when given the unbalanced equation. In order to balance al + o2 = al2o3 you'll need to watch out for two things. It shows the reactants (substances that start a reaction) and products (substances. How do you write the balanced molecular and net ionic equations for the reaction between aluminum. Aluminum Oxide Balanced Equation.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron Aluminum Oxide Balanced Equation Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. A chemical equation represents a chemical reaction. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? It shows the reactants (substances that start a reaction) and products (substances. To balance a chemical equation, enter. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Aluminum reacts with oxygen to produce aluminum oxide based on Aluminum Oxide Balanced Equation Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Aluminum oxide = aluminium + dioxygen. Balance a chemical equation when given the unbalanced equation. In order to balance al + o2 = al2o3 you'll. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced equation to represent the electrolysis of Aluminum Oxide Balanced Equation Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Balance a chemical equation when given the unbalanced equation. In order to balance al + o2 = al2o3 you'll need to watch out for two things. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations for the following reactions Aluminum Oxide Balanced Equation Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance. Aluminum Oxide Balanced Equation.
From www.chegg.com
Solved Aluminum Metal Reacts With Oxygen Gas To Form Alum... Aluminum Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? The balanced equation will appear. Balance a chemical equation when given the unbalanced equation. Al2o3 = al + o2 is a decomposition. Aluminum Oxide Balanced Equation.
From www.meritnation.com
Write balanced chemical equation for Aluminium oxide and sodium oxide Aluminum Oxide Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. The balanced equation will appear. Aluminum oxide = aluminium + dioxygen. A chemical equation represents a chemical reaction. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. In order to balance al + o2 = al2o3 you'll need to watch out. Aluminum Oxide Balanced Equation.
From www.youtube.com
Aluminium Oxide Balanced symbol equation YouTube Aluminum Oxide Balanced Equation The balanced equation will appear. A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. How do you write the balanced molecular and net ionic equations for the reaction between. Aluminum Oxide Balanced Equation.
From www.nagwa.com
Question Video Describing the Oxide of Aluminum Nagwa Aluminum Oxide Balanced Equation A chemical equation represents a chemical reaction. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Explain the roles of subscripts and coefficients in chemical equations. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Write a balanced equation for the. Aluminum Oxide Balanced Equation.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation Aluminum Oxide Balanced Equation A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In order to balance al + o2 = al2o3 you'll need to watch out for two things. How do you write the balanced molecular. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for the formation of aluminum oxide Aluminum Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. In order to balance al + o2 = al2o3 you'll. Aluminum Oxide Balanced Equation.
From brainly.in
3. Write balanced chemical equation.When Aluminium reacts with oxygen Aluminum Oxide Balanced Equation Balance a chemical equation when given the unbalanced equation. A chemical equation represents a chemical reaction. In order to balance al + o2 = al2o3 you'll need to watch out for two things. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Al2o3 = al + o2 is a decomposition reaction. Aluminum Oxide Balanced Equation.
From slideplayer.com
Chapter ppt download Aluminum Oxide Balanced Equation The balanced equation will appear. A chemical equation represents a chemical reaction. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Aluminum oxide = aluminium + dioxygen. To balance a chemical equation, enter an equation of. Aluminum Oxide Balanced Equation.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Aluminum Oxide Balanced Equation A chemical equation represents a chemical reaction. Aluminum oxide = aluminium + dioxygen. Explain the roles of subscripts and coefficients in chemical equations. It shows the reactants (substances that start a reaction) and products (substances. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the. Aluminum Oxide Balanced Equation.
From www.doubtnut.com
Write a balanced equation for the reaction betwee aluminium oxide & s Aluminum Oxide Balanced Equation In order to balance al + o2 = al2o3 you'll need to watch out for two things. Balance a chemical equation when given the unbalanced equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Explain the. Aluminum Oxide Balanced Equation.
From www.youtube.com
Al2O3+H2SO4=Al2(SO4)3+H2O Balanced EquationAluminum oxide+Sulphuric Aluminum Oxide Balanced Equation In order to balance al + o2 = al2o3 you'll need to watch out for two things. A chemical equation represents a chemical reaction. Aluminum oxide = aluminium + dioxygen. Explain the roles of subscripts and coefficients in chemical equations. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. The balanced equation will appear.. Aluminum Oxide Balanced Equation.
From www.youtube.com
008 Aluminum + Oxygen = Aluminum Oxide YouTube Aluminum Oxide Balanced Equation It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction. Balance a chemical equation when given the unbalanced equation. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Explain the roles of subscripts and coefficients in chemical equations. The balanced. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Aluminum metal reacts with aqueous iron(II) oxide to form Aluminum Oxide Balanced Equation Aluminum oxide = aluminium + dioxygen. It shows the reactants (substances that start a reaction) and products (substances. Balance a chemical equation when given the unbalanced equation. A chemical equation represents a chemical reaction. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Write a balanced equation for the. Aluminum Oxide Balanced Equation.
From www.chegg.com
Solved Aluminum reacts with oxygen to produce aluminum oxide Aluminum Oxide Balanced Equation How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? It shows the reactants (substances that start a reaction) and products (substances. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The balanced equation will appear. A chemical equation represents a. Aluminum Oxide Balanced Equation.
From www.slideserve.com
PPT Unit 8 Chemical Reactions PowerPoint Presentation, free download Aluminum Oxide Balanced Equation Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Aluminum oxide = aluminium + dioxygen. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. In order to balance al + o2 = al2o3 you'll need to watch out for two things. The balanced equation. Aluminum Oxide Balanced Equation.
From www.meritnation.com
Ferric oxide reacts with aluminium to produce aluminium oxide and iron Aluminum Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. The balanced equation will appear. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Aluminum oxide = aluminium +. Aluminum Oxide Balanced Equation.
From www.slideserve.com
PPT Equations Review PowerPoint Presentation, free download ID5877618 Aluminum Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Balance a chemical equation when given the unbalanced equation. The balanced equation will appear. Explain the roles of subscripts and coefficients in chemical equations. Aluminum oxide = aluminium + dioxygen. In order to balance al + o2 = al2o3 you'll need to. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Cryolile , Nay AIF6(S) , an Ore used in the production of Aluminum Oxide Balanced Equation How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? It shows the reactants (substances that start a reaction) and products (substances. Aluminum oxide = aluminium + dioxygen. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Balance a chemical equation when given the. Aluminum Oxide Balanced Equation.
From www.numerade.com
SOLVED Aluminum sulfate, Al2(SO4)3, to form aluminum oxide Aluminum Oxide Balanced Equation A chemical equation represents a chemical reaction. Explain the roles of subscripts and coefficients in chemical equations. How do you write the balanced molecular and net ionic equations for the reaction between aluminum metal and silver nitrate? Aluminum oxide = aluminium + dioxygen. In order to balance al + o2 = al2o3 you'll need to watch out for two things.. Aluminum Oxide Balanced Equation.