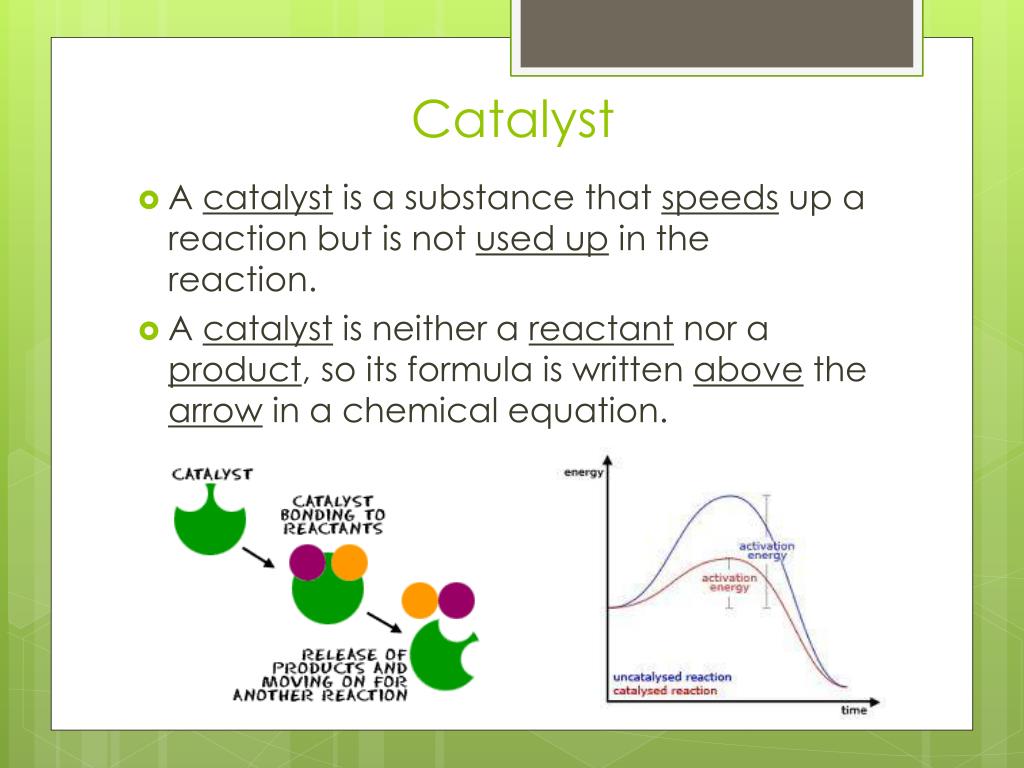
Catalysts Work By . In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysis works by giving chemical reactions that should happen a way to actually happen. Decreased activation energy means less energy required to start the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the. The standard way to think of a chemical reaction. A catalyst increases the rate of reaction by decreasing the activation energy. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee How does a catalyst work?
from www.slideserve.com
How does a catalyst work? Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. In general, catalytic action is a chemical reaction between the catalyst and a reactant. A catalyst increases the rate of reaction by decreasing the activation energy. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Describe the similarities and differences between the. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Decreased activation energy means less energy required to start the reaction.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst increases the rate of reaction by decreasing the activation energy. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. What are catalysts, and how do they work in terms altering the parameters of a reaction? Decreased activation energy means less energy required to start the reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. How does a catalyst work? Describe the similarities and differences between the. The standard way to think of a chemical reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee Catalysis works by giving chemical reactions that should happen a way to actually happen. In general, catalytic action is a chemical reaction between the catalyst and a reactant.
From www.researchgate.net
Schematic diagram of the catalysts preparation procedure Download Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Catalysts Work By.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalysts Work By Decreased activation energy means less energy required to start the reaction. The standard way to think of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst increases the rate of reaction by. Catalysts Work By.
From www.youtube.com
Chapter 14 Chemical Part 6 of 17 YouTube Catalysts Work By Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis works by giving chemical reactions that should happen a way to actually happen. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst increases the rate of reaction by decreasing the activation energy. Catalysts work. Catalysts Work By.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Catalysts Work By A catalyst increases the rate of reaction by decreasing the activation energy. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the. What are catalysts, and how do they work in terms. Catalysts Work By.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 Catalysts Work By What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee The standard way to think of a chemical reaction. When the catalyst is an enzyme, the enzyme. Catalysts Work By.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalysts Work By What are catalysts, and how do they work in terms altering the parameters of a reaction? In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst increases the rate of reaction by decreasing the activation energy. In general, catalytic action is a chemical reaction between the catalyst. Catalysts Work By.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of Catalysts Work By Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. How does a catalyst work? Describe the similarities and differences between the. Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee Decreased activation energy means less energy required to start the. Catalysts Work By.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalysts Work By Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Catalysis works by giving chemical reactions that should happen a way to actually happen. Describe the similarities and differences between the. The standard way to think of a chemical. Catalysts Work By.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Catalysts Work By In general, catalytic action is a chemical reaction between the catalyst and a reactant. How does a catalyst work? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst increases the rate of reaction by decreasing the activation energy. Catalysis works by giving chemical reactions that should happen a way to actually. Catalysts Work By.
From www.catalystseurope.org
What are catalysts? Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an alternative pathway for the. Catalysts Work By.
From scitechdaily.com
Science Made Simple What Are Catalysts? Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Decreased activation energy means less energy. Catalysts Work By.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. The standard way to think of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Decreased activation energy. Catalysts Work By.
From www.youtube.com
How Catalysts Work (GCSE) YouTube Catalysts Work By The standard way to think of a chemical reaction. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. What are catalysts, and how do they work in terms altering the parameters of a reaction? When the catalyst is an enzyme, the enzyme binds to a substrate, leading to. Catalysts Work By.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Catalysts Work By A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Describe the similarities and differences between the. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an alternative pathway for the reaction to occur that requires. Catalysts Work By.
From slideplayer.com
How Catalysts Work Main Concept ppt download Catalysts Work By The standard way to think of a chemical reaction. How does a catalyst work? What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to. Catalysts Work By.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Work By What are catalysts, and how do they work in terms altering the parameters of a reaction? Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The standard way to think of a chemical reaction. Describe the similarities and differences between the. How does a catalyst work? In chemistry, a catalyst is a substance that increases the rate of. Catalysts Work By.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID5408111 Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The standard way to think of a chemical. Catalysts Work By.
From www.savemyexams.com
Catalysts AQA GCSE Chemistry Revision Notes 2018 Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. How does a catalyst work? A catalyst. Catalysts Work By.
From www.catalystseurope.org
How are catalysts used? Catalysts Work By How does a catalyst work? In general, catalytic action is a chemical reaction between the catalyst and a reactant. The standard way to think of a chemical reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. When the catalyst is an enzyme, the enzyme binds. Catalysts Work By.
From www.slideserve.com
PPT CATALYSIS A guide for A level students PowerPoint Presentation Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. How does a catalyst work? Describe the similarities and differences between the. In general, catalytic action is a chemical reaction between the catalyst and a reactant. A catalyst binds to a reactant and it increases the number of collision. Catalysts Work By.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst Catalysts Work By When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. The standard way to think of a chemical reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. How does a catalyst work? Describe the similarities and differences between the. In chemistry, a catalyst is a substance that increases the. Catalysts Work By.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalysts Work By The standard way to think of a chemical reaction. A catalyst increases the rate of reaction by decreasing the activation energy. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. How does a catalyst work? A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the. Catalysts Work By.
From ch302.cm.utexas.edu
chem1 Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. What are catalysts, and how do they work in terms altering the parameters of a reaction?. Catalysts Work By.
From gradegorilla.com
Gradegorilla Chemistry Catalysts Work By The standard way to think of a chemical reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. What are catalysts, and how do they. Catalysts Work By.
From online-learning-college.com
Catalysts What is a catalyst? How do they work? Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. How does a catalyst work? What are catalysts, and how do they work in terms altering the parameters of a reaction? Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The standard way to think of a. Catalysts Work By.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalysts Work By In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. What are catalysts, and how do they work in terms altering the parameters of. Catalysts Work By.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2229930 Catalysts Work By The standard way to think of a chemical reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. What are catalysts, and how do they work in. Catalysts Work By.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalysts Work By How does a catalyst work? A catalyst increases the rate of reaction by decreasing the activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee. Catalysts Work By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Work By Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee The standard way to think of a chemical reaction. Decreased activation energy means less energy required to start the reaction. A catalyst binds to a reactant and it increases the number of collision. Catalysts Work By.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts Catalysts Work By A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. How does a catalyst work? Catalysts work by providing an alternative pathway for the reaction to occur that requires less energy, which lowers the activation energy required for the reaction to procee Decreased activation energy means less. Catalysts Work By.
From www.youtube.com
How does a CATALYST work ? YouTube Catalysts Work By Catalysis works by giving chemical reactions that should happen a way to actually happen. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Describe the similarities and differences between the. How does a catalyst work? Decreased activation energy means less energy required to start the reaction. In chemistry, a catalyst is a substance that. Catalysts Work By.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download Catalysts Work By The standard way to think of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. In chemistry, a catalyst is a substance that increases. Catalysts Work By.
From www.chim.lu
Activation energy and catalysis Catalysts Work By When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. Catalysis works by giving chemical reactions that should happen a way to actually happen. The standard way to think of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry, a catalyst is a. Catalysts Work By.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts Work By How does a catalyst work? When the catalyst is an enzyme, the enzyme binds to a substrate, leading to catalysis. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. What are catalysts, and how do they work. Catalysts Work By.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Work By Describe the similarities and differences between the. How does a catalyst work? In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. In general, catalytic action. Catalysts Work By.