How To Use Dilution Formula . For both sorts of dilution factors, the. Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. To dilute a solution means to add more solvent without the addition of more solute. C 1 v 1 = c 2 v 2 where: Of course, the resulting solution is thoroughly mixed so as to. In any dilution, the number of moles of solute stays the same. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: For diluting solutions in lab experiments, the formal formula for calculating. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You use the formula #v_1c_1 = v_2c_2#.
from www.youtube.com
To dilute a solution means to add more solvent without the addition of more solute. For diluting solutions in lab experiments, the formal formula for calculating. Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Of course, the resulting solution is thoroughly mixed so as to. In any dilution, the number of moles of solute stays the same.
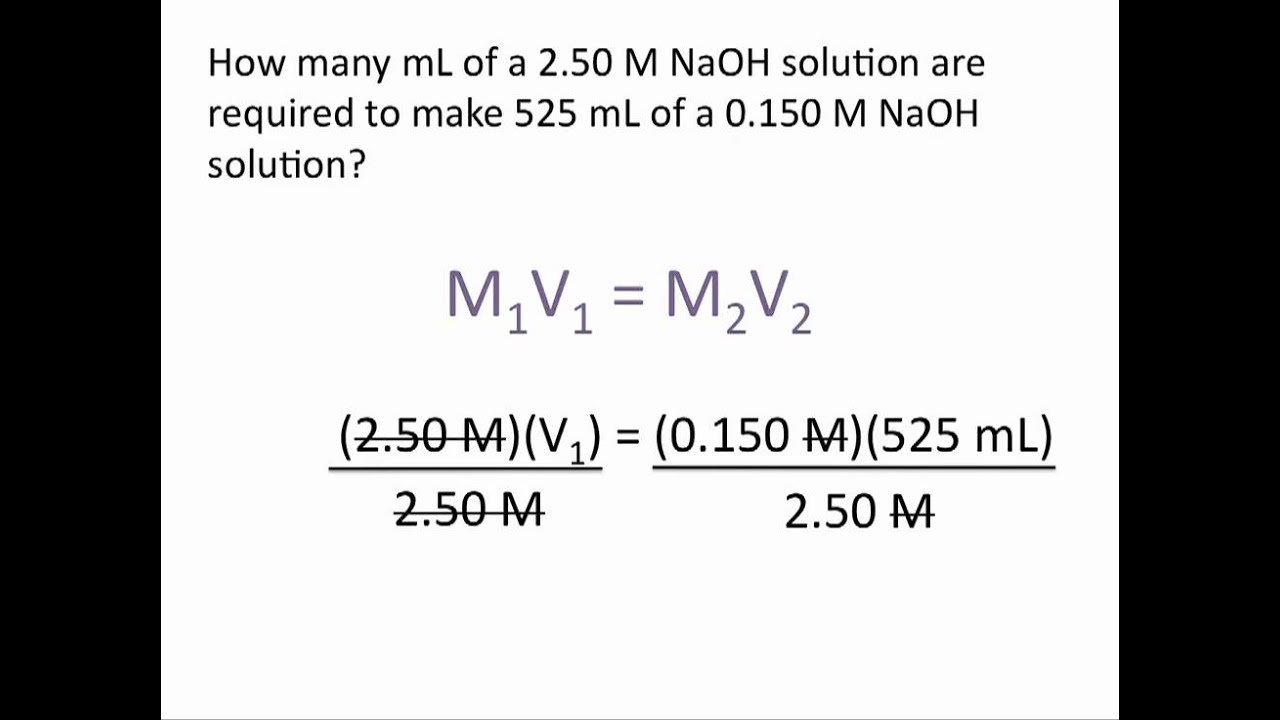
Dilution Problems Chemistry Tutorial YouTube
How To Use Dilution Formula Learn how to dilute and concentrate solutions. For both sorts of dilution factors, the. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. You use the formula #v_1c_1 = v_2c_2#. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. In any dilution, the number of moles of solute stays the same. To dilute a solution means to add more solvent without the addition of more solute. For diluting solutions in lab experiments, the formal formula for calculating.
From www.slideshare.net
Molarity and dilution How To Use Dilution Formula Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You use the formula #v_1c_1 = v_2c_2#. C 1 v 1 = c 2 v 2 where: In any dilution, the number of moles of solute stays the same. State whether the concentration of a solution is directly or indirectly proportional to. How To Use Dilution Formula.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube How To Use Dilution Formula For both sorts of dilution factors, the. For diluting solutions in lab experiments, the formal formula for calculating. You use the formula #v_1c_1 = v_2c_2#. C 1 v 1 = c 2 v 2 where: In any dilution, the number of moles of solute stays the same. State whether the concentration of a solution is directly or indirectly proportional to. How To Use Dilution Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free How To Use Dilution Formula For both sorts of dilution factors, the. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Dilution is the addition of. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. In any dilution, the number. How To Use Dilution Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free How To Use Dilution Formula You use the formula #v_1c_1 = v_2c_2#. In any dilution, the number of moles of solute stays the same. For both sorts of dilution factors, the. Of course, the resulting solution is thoroughly mixed so as to. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. C 1 v 1 = c 2. How To Use Dilution Formula.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID How To Use Dilution Formula Dilution is the addition of. C 1 v 1 = c 2 v 2 where: State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To dilute a solution means to add more solvent without the addition. How To Use Dilution Formula.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration How To Use Dilution Formula To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. You use the formula #v_1c_1 = v_2c_2#. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Often, a worker will need to change the concentration. How To Use Dilution Formula.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii How To Use Dilution Formula To make a fixed amount of a dilute solution from a stock solution, you can use the formula: To dilute a solution means to add more solvent without the addition of more solute. You use the formula #v_1c_1 = v_2c_2#. Learn how to dilute and concentrate solutions. Dilution is the addition of. In any dilution, the number of moles of. How To Use Dilution Formula.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation How To Use Dilution Formula Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Often, a worker will. How To Use Dilution Formula.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) How To Use Dilution Formula For both sorts of dilution factors, the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is. How To Use Dilution Formula.
From www.youtube.com
Video 18 Simple C1V1 = C2V2 dilution calculations YouTube How To Use Dilution Formula Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Of course, the resulting solution is thoroughly mixed so as to. To make a. How To Use Dilution Formula.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate How To Use Dilution Formula You use the formula #v_1c_1 = v_2c_2#. For diluting solutions in lab experiments, the formal formula for calculating. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use. How To Use Dilution Formula.
From www.youtube.com
Chem143 Dilution Equation YouTube How To Use Dilution Formula For diluting solutions in lab experiments, the formal formula for calculating. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Of course, the resulting solution is thoroughly mixed so as to. State whether. How To Use Dilution Formula.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free How To Use Dilution Formula Learn how to dilute and concentrate solutions. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. In any dilution, the number of moles of solute stays the same. To make a fixed amount of a dilute solution from. How To Use Dilution Formula.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube How To Use Dilution Formula To make a fixed amount of a dilute solution from a stock solution, you can use the formula: State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. You use the formula #v_1c_1 = v_2c_2#. In any dilution, the number of moles of solute stays the same. Of. How To Use Dilution Formula.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew How To Use Dilution Formula Often, a worker will need to change the concentration of a solution by changing the amount of solvent. C 1 v 1 = c 2 v 2 where: Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. In any dilution, the number of moles of solute. How To Use Dilution Formula.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved How To Use Dilution Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Dilution is the addition of. For both sorts of dilution factors, the. Learn how to dilute and concentrate solutions. You use the formula #v_1c_1 = v_2c_2#. For diluting solutions. How To Use Dilution Formula.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How To Use Dilution Formula Dilution is the addition of. For diluting solutions in lab experiments, the formal formula for calculating. To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing. How To Use Dilution Formula.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution How To Use Dilution Formula Learn how to dilute and concentrate solutions. Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. For both sorts of dilution factors, the. For diluting solutions in lab experiments, the formal formula for calculating. To make a fixed amount of a dilute solution from a stock solution, you can. How To Use Dilution Formula.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download How To Use Dilution Formula Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You use the formula #v_1c_1 = v_2c_2#. To dilute a solution means to add more solvent without the addition of more solute. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Of course, the resulting. How To Use Dilution Formula.
From www.youtube.com
Dilution Calculation Practice YouTube How To Use Dilution Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Dilution is the addition of. In any dilution, the number of moles of solute stays the same. Dilution factor formula (equation) as previously stated, the dilution factor. How To Use Dilution Formula.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID How To Use Dilution Formula To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. For both sorts of dilution factors, the. C 1 v 1 = c 2. How To Use Dilution Formula.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID How To Use Dilution Formula To dilute a solution means to add more solvent without the addition of more solute. You use the formula #v_1c_1 = v_2c_2#. Dilution is the addition of. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: State whether the concentration of a solution is directly or indirectly proportional to its volume.. How To Use Dilution Formula.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples How To Use Dilution Formula To dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. In any dilution, the number of moles of solute stays the same. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution factor formula (equation) as previously stated, the dilution factor is. How To Use Dilution Formula.
From cfmuvi.jimdo.com
Simple Serial Dilution Calculation cfmuvi How To Use Dilution Formula For both sorts of dilution factors, the. Dilution is the addition of. To dilute a solution means to add more solvent without the addition of more solute. In any dilution, the number of moles of solute stays the same. For diluting solutions in lab experiments, the formal formula for calculating. Often, a worker will need to change the concentration of. How To Use Dilution Formula.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe How To Use Dilution Formula Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: You use the formula #v_1c_1 = v_2c_2#. C 1 v 1 = c 2 v 2. How To Use Dilution Formula.
From www.youtube.com
How to Calculate Dilution Factor YouTube How To Use Dilution Formula Of course, the resulting solution is thoroughly mixed so as to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To dilute a solution means to add more solvent without the addition of more solute. In any dilution, the number of moles of solute stays the same. C 1 v 1. How To Use Dilution Formula.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube How To Use Dilution Formula You use the formula #v_1c_1 = v_2c_2#. For diluting solutions in lab experiments, the formal formula for calculating. To dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. Of course, the resulting. How To Use Dilution Formula.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript How To Use Dilution Formula You use the formula #v_1c_1 = v_2c_2#. Dilution is the addition of. C 1 v 1 = c 2 v 2 where: Of course, the resulting solution is thoroughly mixed so as to. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Often, a worker will need to change the concentration. How To Use Dilution Formula.
From chem2u.blogspot.com
chem2U Dilution method formula How To Use Dilution Formula Learn how to dilute and concentrate solutions. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. You use the formula #v_1c_1 = v_2c_2#. Of course, the resulting solution is thoroughly mixed so as to. In any dilution, the number of moles of solute stays the same. Often, a worker will need to change. How To Use Dilution Formula.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions How To Use Dilution Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. For both sorts of dilution factors, the. To dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. In any dilution, the number. How To Use Dilution Formula.
From www.youtube.com
120 Dilution.Two easy methods to prepare.learn & understand then can How To Use Dilution Formula State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. For diluting solutions in lab experiments, the formal formula for calculating. In any dilution, the number of moles of solute stays the same. C 1 v 1. How To Use Dilution Formula.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How To Use Dilution Formula In any dilution, the number of moles of solute stays the same. C 1 v 1 = c 2 v 2 where: Dilution is the addition of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.. How To Use Dilution Formula.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How To Use Dilution Formula Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. For both sorts of dilution factors, the. Dilution is the addition of. For diluting solutions in lab experiments, the formal formula for calculating. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: In any dilution,. How To Use Dilution Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free How To Use Dilution Formula For diluting solutions in lab experiments, the formal formula for calculating. To dilute a solution means to add more solvent without the addition of more solute. Dilution factor formula (equation) as previously stated, the dilution factor is frequently given as a ratio. To make a fixed amount of a dilute solution from a stock solution, you can use the formula:. How To Use Dilution Formula.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How To Use Dilution Formula Learn how to dilute and concentrate solutions. In any dilution, the number of moles of solute stays the same. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. To make a fixed amount of a dilute solution from a. How To Use Dilution Formula.