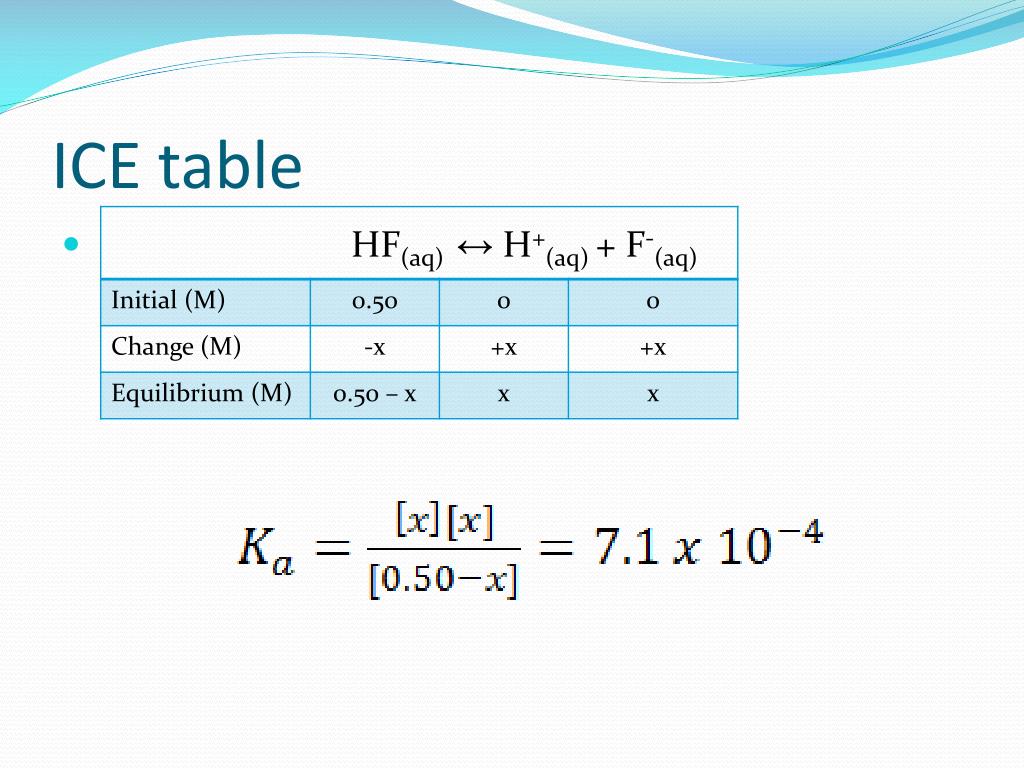
Set Up Ice Table Chemistry . 1) set up a row with the known concentrations of each. Calculating equilibrium constants, k, using a r.i.c.e. To set up an ice table using the following steps. The following reaction has been studied at 25c: Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students I stands for the initial concentrations (or pressures) for each species in the reaction mixture. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from.
from www.slideserve.com
Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An useful tool in solving equilibrium problems is an ice chart. 1) set up a row with the known concentrations of each. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. To set up an ice table using the following steps. Table, a tutorial with worked examples suitable for chemistry students An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. The following reaction has been studied at 25c: Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial concentrations (or pressures) for each species in the reaction mixture.
PPT Ka, Kb PowerPoint Presentation, free download ID3060507
Set Up Ice Table Chemistry Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. The following reaction has been studied at 25c: Calculating equilibrium constants, k, using a r.i.c.e. Table, a tutorial with worked examples suitable for chemistry students An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. To set up an ice table using the following steps. An useful tool in solving equilibrium problems is an ice chart. 1) set up a row with the known concentrations of each. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc YouTube Set Up Ice Table Chemistry 1) set up a row with the known concentrations of each. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice table is used for determining the equilibrium concentrations based on. Set Up Ice Table Chemistry.
From general.chemistrysteps.com
pH of a Weak Acid Chemistry Steps Set Up Ice Table Chemistry An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students 1) set up a row with the known concentrations of each. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial. Set Up Ice Table Chemistry.
From www.youtube.com
Chemical Equilibrium ICE Table Example 3 YouTube Set Up Ice Table Chemistry 1) set up a row with the known concentrations of each. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating. Set Up Ice Table Chemistry.
From www.youtube.com
equilibrium ICE tables YouTube Set Up Ice Table Chemistry An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. The following reaction has been studied at 25c: To set up an ice table using the following steps. 1) set up a row. Set Up Ice Table Chemistry.
From www.youtube.com
Extent of Reaction (ξ) ICE Tables in Physical Chemistry YouTube Set Up Ice Table Chemistry Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Table, a tutorial with worked examples suitable for chemistry students Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. 1) set up a row with the known. Set Up Ice Table Chemistry.
From www.conquerhsc.com
HSC Chemistry Module 5 Inquiry Question 3 Set Up Ice Table Chemistry An useful tool in solving equilibrium problems is an ice chart. Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Ice tables automatically set up and organize. Set Up Ice Table Chemistry.
From www.youtube.com
Solving Equilibrium ICE Tables WITHOUT the Quadratic Formula YouTube Set Up Ice Table Chemistry Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. 1) set up a row with the known concentrations of each. To set up an ice table using the following steps. The following reaction has been studied at 25c: I stands for the initial concentrations (or pressures) for each species. Set Up Ice Table Chemistry.
From www.youtube.com
General Chemistry III Extra ICETable Practice 1 of 2 YouTube Set Up Ice Table Chemistry To set up an ice table using the following steps. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Table, a tutorial with worked examples suitable for chemistry students Calculating equilibrium constants, k, using a r.i.c.e. Ice tables automatically set up and organize the variables and constants. Set Up Ice Table Chemistry.
From www.chegg.com
Not understanding how to make the ICE table for each Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. To set up an ice table using the following steps. Table, a tutorial with worked examples suitable for chemistry students Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Calculating equilibrium constants, k, using a r.i.c.e. An ice. Set Up Ice Table Chemistry.
From hxeyiqaec.blob.core.windows.net
Chemistry Ice Table Calculator at Rhonda Villarreal blog Set Up Ice Table Chemistry The following reaction has been studied at 25c: An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Calculating equilibrium constants, k, using a r.i.c.e. Table, a tutorial with worked examples suitable for chemistry students Ice tables automatically set up and organize the variables and constants needed when. Set Up Ice Table Chemistry.
From www.youtube.com
Chemical Equilibrium V The ICE Table Introduction YouTube Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Calculating equilibrium constants, k, using a r.i.c.e. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked. Set Up Ice Table Chemistry.
From solovelytogether.blogspot.com
How To Do Ice Tables Chemistry Decoration Drawing Set Up Ice Table Chemistry An useful tool in solving equilibrium problems is an ice chart. Calculating equilibrium constants, k, using a r.i.c.e. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Table, a tutorial with worked examples suitable for chemistry students The following reaction has been studied at 25c: I stands for the initial concentrations (or pressures). Set Up Ice Table Chemistry.
From www.youtube.com
Chemistry Tutorial and Examples of ICE Tables YouTube Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An useful tool in solving equilibrium problems is an ice chart. Calculating equilibrium constants, k, using a r.i.c.e. An ice table (for initial concentration, change. Set Up Ice Table Chemistry.
From www.youtube.com
Reaction quotients and ICE tables YouTube Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. 1) set up a row with the known concentrations of each. An useful tool in solving equilibrium problems is an ice chart. To set up an ice table using the following steps. Calculating equilibrium constants, k, using a r.i.c.e. The following reaction has been studied. Set Up Ice Table Chemistry.
From www.reddit.com
(HS Chemistry) 23 How do you set up the ICE table when you add Set Up Ice Table Chemistry An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Calculating equilibrium constants, k, using a r.i.c.e. I stands for the initial concentrations (or pressures) for each species in the reaction mixture.. Set Up Ice Table Chemistry.
From www.youtube.com
Equilibrium Reaction with an ICE Table Chemistry Sample Problem YouTube Set Up Ice Table Chemistry The following reaction has been studied at 25c: 1) set up a row with the known concentrations of each. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Calculating equilibrium constants, k, using a r.i.c.e. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a. Set Up Ice Table Chemistry.
From www.showme.com
Ice table intro Science, Chemistry, Equilibrium ShowMe Set Up Ice Table Chemistry An useful tool in solving equilibrium problems is an ice chart. 1) set up a row with the known concentrations of each. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. To set up an ice table using the following steps. Calculating equilibrium constants, k, using a. Set Up Ice Table Chemistry.
From www.youtube.com
Chemical Equilibrium Ice Table Equilibrium Constant Expression Set Up Ice Table Chemistry To set up an ice table using the following steps. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students An ice table (for initial concentration, change in concentration, and equilibrium concentration) is. Set Up Ice Table Chemistry.
From www.pinterest.com.mx
Chemistry wedding centerpieces. Glassware, colored water, and dry ice Set Up Ice Table Chemistry Table, a tutorial with worked examples suitable for chemistry students Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An useful tool in solving equilibrium problems is an ice chart. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an. Set Up Ice Table Chemistry.
From naithanteggan.blogspot.com
17+ Ice Table Chemistry NaithanTeggan Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students The following reaction has. Set Up Ice Table Chemistry.
From studylibmayer.z13.web.core.windows.net
Ice Table Chemistry Worksheet Set Up Ice Table Chemistry Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Calculating equilibrium constants, k, using a r.i.c.e. To set up an ice table using the following steps. The following reaction has been studied at. Set Up Ice Table Chemistry.
From www.youtube.com
Chemical Equilibrium ICE Table Example 2 YouTube Set Up Ice Table Chemistry The following reaction has been studied at 25c: An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students Calculating equilibrium constants, k, using a r.i.c.e. 1) set up a row with the known concentrations of each. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is. Set Up Ice Table Chemistry.
From www.slideserve.com
PPT Ka, Kb PowerPoint Presentation, free download ID3060507 Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An useful tool in solving equilibrium problems is an ice chart. The following reaction has been studied at 25c: Ice table is used for determining the equilibrium concentrations based. Set Up Ice Table Chemistry.
From www.youtube.com
Chem 2 Unit 9 ICE Tables and Q YouTube Set Up Ice Table Chemistry Calculating equilibrium constants, k, using a r.i.c.e. An useful tool in solving equilibrium problems is an ice chart. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. 1) set up a row with the known concentrations of each. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and. Set Up Ice Table Chemistry.
From www.reddit.com
[Grade 10 Chemistry Common Ion Effect] How to set up ICE table? r Set Up Ice Table Chemistry Calculating equilibrium constants, k, using a r.i.c.e. The following reaction has been studied at 25c: An useful tool in solving equilibrium problems is an ice chart. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an. Set Up Ice Table Chemistry.
From www.youtube.com
ICE Tables and Quadratic Formula, ICE Tables and Small Equilibrium Set Up Ice Table Chemistry 1) set up a row with the known concentrations of each. The following reaction has been studied at 25c: I stands for the initial concentrations (or pressures) for each species in the reaction mixture. An useful tool in solving equilibrium problems is an ice chart. Calculating equilibrium constants, k, using a r.i.c.e. Ice table is used for determining the equilibrium. Set Up Ice Table Chemistry.
From studyfinder.org
Ice Table Chemistry Practice Problems Unlocking Answers for Better Set Up Ice Table Chemistry Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students The following reaction has been studied at 25c:. Set Up Ice Table Chemistry.
From www.youtube.com
ICE Tables and Quadratic Formula, ICE Tables and Small Equilibrium Set Up Ice Table Chemistry The following reaction has been studied at 25c: Calculating equilibrium constants, k, using a r.i.c.e. Table, a tutorial with worked examples suitable for chemistry students 1) set up a row with the known concentrations of each. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. I stands for the. Set Up Ice Table Chemistry.
From www.youtube.com
How to find equilibrium concentrations using an ICE table YouTube Set Up Ice Table Chemistry 1) set up a row with the known concentrations of each. To set up an ice table using the following steps. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. An useful tool in solving equilibrium problems is an ice chart. The following reaction has been studied at 25c: Calculating equilibrium constants, k,. Set Up Ice Table Chemistry.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube Set Up Ice Table Chemistry An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. Calculating equilibrium constants, k, using a r.i.c.e. Table, a tutorial with worked examples suitable for chemistry students 1). Set Up Ice Table Chemistry.
From www.youtube.com
InitialChangeEquilibrium (ICE) Tables YouTube Set Up Ice Table Chemistry Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. I stands for the initial concentrations (or pressures) for each species in the reaction mixture. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. 1) set up a row with the known concentrations. Set Up Ice Table Chemistry.
From www.slideserve.com
PPT Unit 4 Equilibrium PowerPoint Presentation, free download ID Set Up Ice Table Chemistry I stands for the initial concentrations (or pressures) for each species in the reaction mixture. An ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Table, a tutorial with worked examples suitable for chemistry students 1) set up a row with the known concentrations of each. The following. Set Up Ice Table Chemistry.
From www.youtube.com
Perfect Squares ICE Table Equilibrium Calculations YouTube Set Up Ice Table Chemistry Calculating equilibrium constants, k, using a r.i.c.e. An useful tool in solving equilibrium problems is an ice chart. Table, a tutorial with worked examples suitable for chemistry students I stands for the initial concentrations (or pressures) for each species in the reaction mixture. 1) set up a row with the known concentrations of each. The following reaction has been studied. Set Up Ice Table Chemistry.
From www.youtube.com
Equilibrium 5.4 ICE Tables YouTube Set Up Ice Table Chemistry Calculating equilibrium constants, k, using a r.i.c.e. Ice tables automatically set up and organize the variables and constants needed when calculating the unknown. To set up an ice table using the following steps. An useful tool in solving equilibrium problems is an ice chart. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the. Set Up Ice Table Chemistry.
From www.chegg.com
Solved Set up ICE tables for Mixtures A and B, then Set Up Ice Table Chemistry Table, a tutorial with worked examples suitable for chemistry students To set up an ice table using the following steps. Ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and reacted concentrations. 1) set up a row with the known concentrations of each. The following reaction has been studied at 25c: Ice. Set Up Ice Table Chemistry.