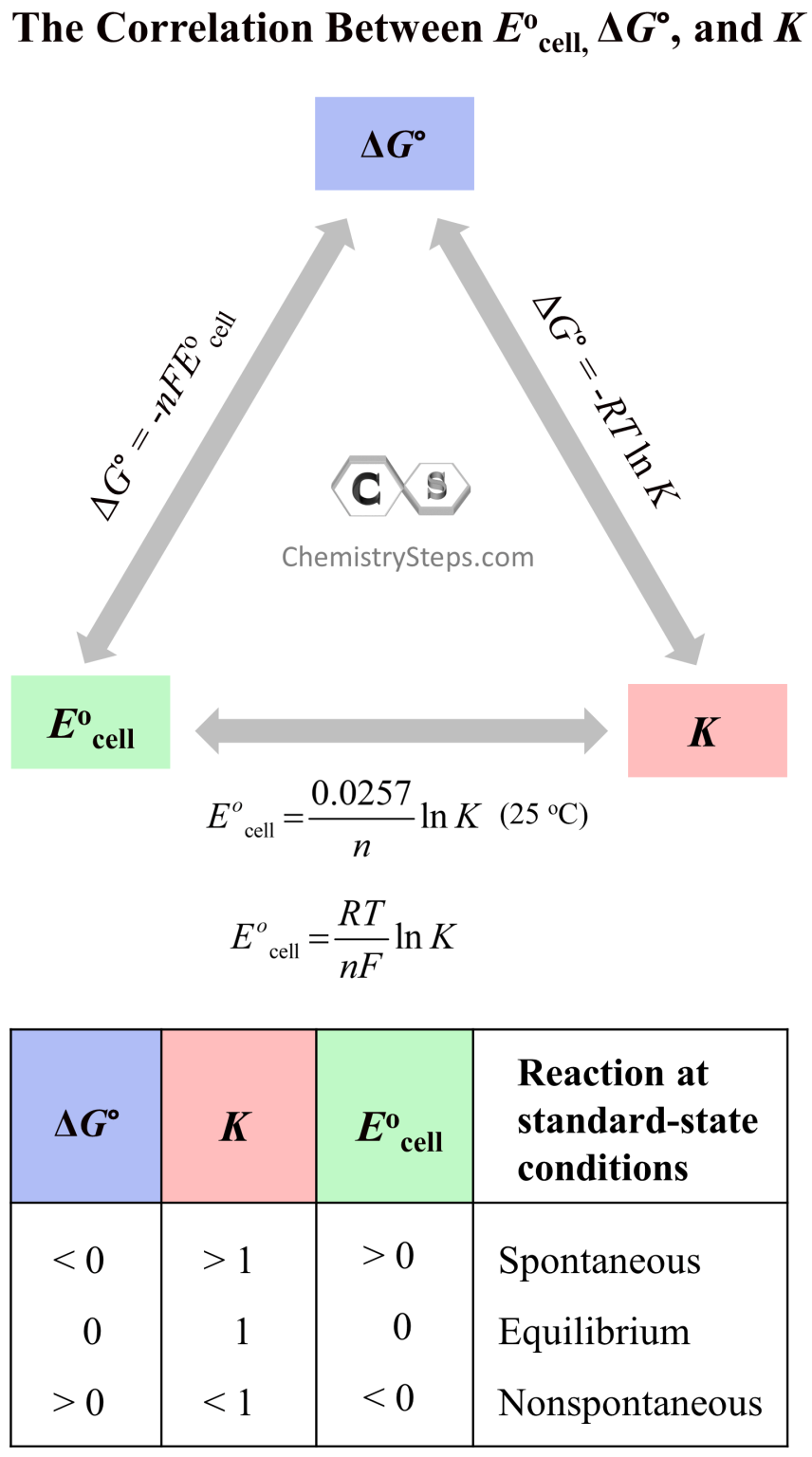
Standard Cell Potential Diagram . The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell.
from general.chemistrysteps.com
The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps
Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods:
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Standard Cell Potential Diagram The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known. Standard Cell Potential Diagram.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Diagram The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The emf measured when a metal / metal. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by subtracting. Standard Cell Potential Diagram.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The cell potential, \(e_{cell}\), is the measure of the. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids. Standard Cell Potential Diagram.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf measured when a metal / metal ion electrode. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chemistry 30 Chapter 14 PowerPoint Presentation, free download Standard Cell Potential Diagram The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The potential of the cell under standard. Standard Cell Potential Diagram.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard cell potential (e 0 cell) is. Standard Cell Potential Diagram.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Diagram The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell.. Standard Cell Potential Diagram.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined. Standard Cell Potential Diagram.
From www.chegg.com
Solved C. Calculating Standard Cell Potentials, Eºcell Given Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is. Standard Cell Potential Diagram.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. This is also known as the standard cell potential. Standard Cell Potential Diagram.
From userdiagramforsyth.z21.web.core.windows.net
Electric Potential Cell Diagram Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell. Standard Cell Potential Diagram.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. This is also known as the standard cell. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell. Standard Cell Potential Diagram.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The cell potential, \(e_{cell}\), is the measure of. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chapter 12 OxidationReduction Reactions PowerPoint Presentation Standard Cell Potential Diagram The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential. Standard Cell Potential Diagram.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Standard Cell Potential Diagram The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (e 0. Standard Cell Potential Diagram.
From courses.lumenlearning.com
Resting Membrane Potential Biology for Majors II Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The cell potential, \(e_{cell}\), is the measure of the. Standard Cell Potential Diagram.
From users.highland.edu
Standard Potentials Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The emf measured when a metal / metal. Standard Cell Potential Diagram.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard cell potential (ecellꝋ) can be. Standard Cell Potential Diagram.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Lets Set The Stage PowerPoint Presentation, free download ID Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two. Standard Cell Potential Diagram.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e. Standard Cell Potential Diagram.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical. Standard Cell Potential Diagram.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an. Standard Cell Potential Diagram.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The cell potential, \(e_{cell}\), is the measure of. Standard Cell Potential Diagram.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Cell Potential Diagram The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The emf measured when a metal / metal ion electrode. Standard Cell Potential Diagram.
From wisc.pb.unizin.org
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021 Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Diagram.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Diagram The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The emf measured when a metal / metal ion. Standard Cell Potential Diagram.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Diagram The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. This is also known as the standard cell. Standard Cell Potential Diagram.
From www.slideserve.com
PPT REDOX REACTIONS PowerPoint Presentation, free download ID4348855 Standard Cell Potential Diagram The standard cell potential (e 0 cell) is the difference between the two electrodes that generates the voltage in the cell. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (ecellꝋ) can be calculated by subtracting the less positive eꝋ from the more positive eꝋ value.. Standard Cell Potential Diagram.