Why Do Atoms Need 8 Valence Electrons . Atoms generally do not need 8 electrons to stabilize. However, there are three general exceptions to the octet rule: Molecules in which one or more atoms possess more than. Using quantum physics and its models of atoms and bonds we can define. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). Molecules, such as no, with an odd number of electrons; The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell.
from www.chem.ucla.edu
Atoms generally do not need 8 electrons to stabilize. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). Molecules, such as no, with an odd number of electrons; Using quantum physics and its models of atoms and bonds we can define. The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. However, there are three general exceptions to the octet rule: This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. Molecules in which one or more atoms possess more than.
Illustrated Glossary of Organic Chemistry Octet rule
Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. However, there are three general exceptions to the octet rule: Molecules, such as no, with an odd number of electrons; Atoms generally do not need 8 electrons to stabilize. This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. Using quantum physics and its models of atoms and bonds we can define. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Molecules in which one or more atoms possess more than. The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals).
From slideplayer.com
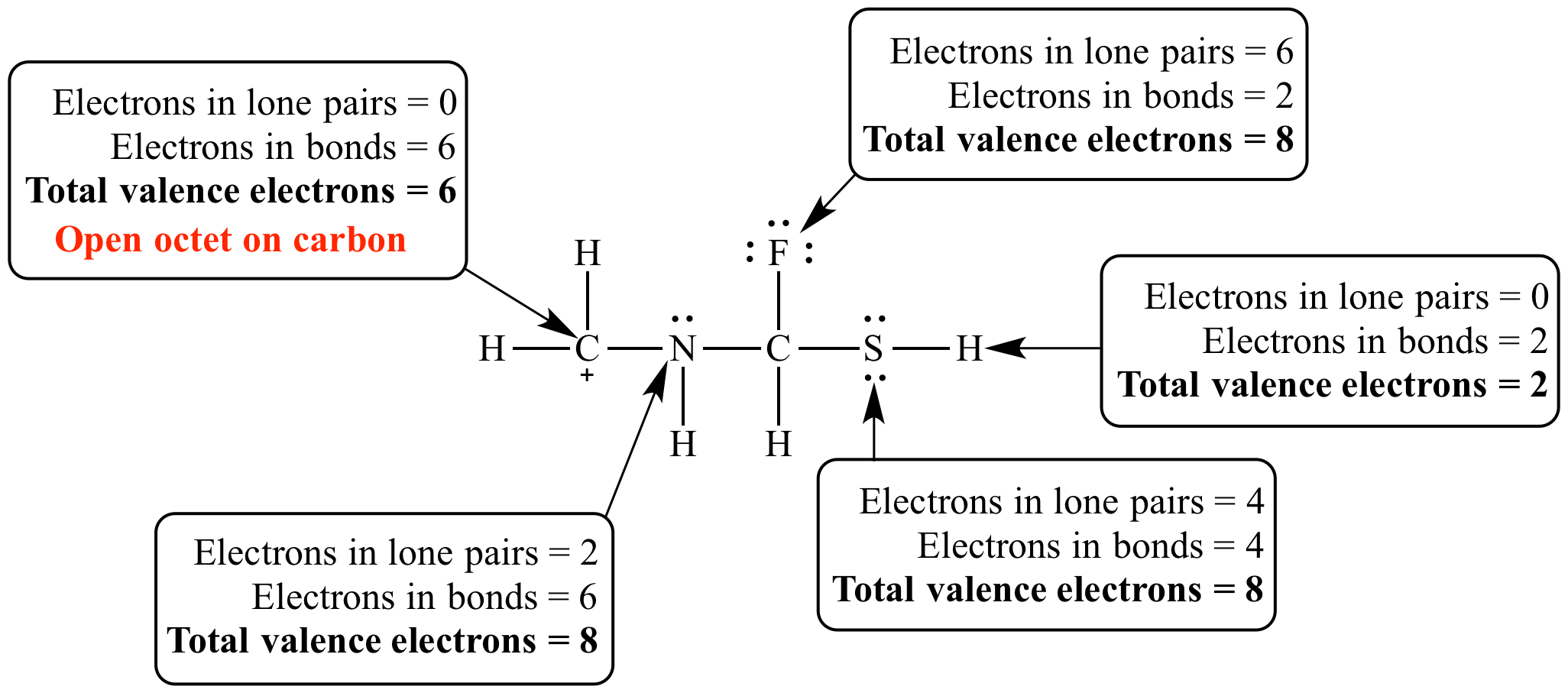
IONIC BONDS Chapter 4 Section ppt download Why Do Atoms Need 8 Valence Electrons This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. Using quantum physics and its models of atoms and bonds we can define. Molecules in which one or more atoms possess more than. However, there are three general exceptions to the octet rule:. Why Do Atoms Need 8 Valence Electrons.
From mavink.com
Valence Electron Orbital Diagram Why Do Atoms Need 8 Valence Electrons Molecules in which one or more atoms possess more than. This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. Using quantum physics and its models of atoms and bonds we can define. The octet rule states that atoms tend to prefer having. Why Do Atoms Need 8 Valence Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Why Do Atoms Need 8 Valence Electrons The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule states that atoms tend to prefer having 8. Why Do Atoms Need 8 Valence Electrons.
From valenceelectrons.com
Valence Electrons Of All Elements Valence Electrons Why Do Atoms Need 8 Valence Electrons The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. The 8 valence electron rule is based on the observation. Why Do Atoms Need 8 Valence Electrons.
From study.com
Valence Electrons Definition, Role & Examples Video & Lesson Why Do Atoms Need 8 Valence Electrons However, there are three general exceptions to the octet rule: Atoms generally do not need 8 electrons to stabilize. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The octet rule refers to the tendency of atoms to prefer to. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
Chemistry Review Chapter 2 in Text. ppt download Why Do Atoms Need 8 Valence Electrons Using quantum physics and its models of atoms and bonds we can define. However, there are three general exceptions to the octet rule: Molecules in which one or more atoms possess more than. Molecules, such as no, with an odd number of electrons; Atoms generally do not need 8 electrons to stabilize. The octet rule refers to the tendency of. Why Do Atoms Need 8 Valence Electrons.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. However, there are three general exceptions to the octet rule: Molecules in which one or more atoms possess more than. Molecules, such as no, with an odd number of electrons; The octet. Why Do Atoms Need 8 Valence Electrons.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? Why Do Atoms Need 8 Valence Electrons The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The. Why Do Atoms Need 8 Valence Electrons.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements Why Do Atoms Need 8 Valence Electrons The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. Molecules in which one or more atoms possess more than. Molecules,. Why Do Atoms Need 8 Valence Electrons.
From praxilabs.com
Your Step by Step Guide to Find Valence Electrons praxilabs Why Do Atoms Need 8 Valence Electrons Using quantum physics and its models of atoms and bonds we can define. Molecules, such as no, with an odd number of electrons; However, there are three general exceptions to the octet rule: The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight. Why Do Atoms Need 8 Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. Molecules in which one or more atoms possess more than. Using quantum physics and its models of atoms and bonds we can define. Molecules, such as no, with an odd number of electrons; This is a consequence of the fact that many compounds involve the s and p block electrons, which. Why Do Atoms Need 8 Valence Electrons.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. However, there are three general exceptions to the octet rule: Molecules, such as no, with an odd number of electrons; Using quantum physics and its models of atoms and bonds we can define. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order. Why Do Atoms Need 8 Valence Electrons.
From www.slideserve.com
PPT Valence Electrons PowerPoint Presentation, free download ID6712840 Why Do Atoms Need 8 Valence Electrons Molecules, such as no, with an odd number of electrons; Using quantum physics and its models of atoms and bonds we can define. However, there are three general exceptions to the octet rule: This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons.. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
Valence Electrons Atoms want to be stable. ppt download Why Do Atoms Need 8 Valence Electrons This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). The octet rule states that atoms tend to prefer having. Why Do Atoms Need 8 Valence Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Why Do Atoms Need 8 Valence Electrons The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. Molecules, such as no, with an odd number of electrons; The octet rule states that atoms tend to. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
UNIT 2 Matter and Atoms. ppt download Why Do Atoms Need 8 Valence Electrons Molecules in which one or more atoms possess more than. Molecules, such as no, with an odd number of electrons; The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Using quantum physics and its models of atoms and bonds we. Why Do Atoms Need 8 Valence Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Why Do Atoms Need 8 Valence Electrons Molecules, such as no, with an odd number of electrons; Molecules in which one or more atoms possess more than. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). The octet rule refers to the tendency of atoms to prefer to have eight electrons in the. Why Do Atoms Need 8 Valence Electrons.
From www.slideserve.com
PPT Intro to Chemistry Ionic Bonds, Covalent Bonds, and Why Do Atoms Need 8 Valence Electrons Molecules, such as no, with an odd number of electrons; The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Using quantum physics and its models of atoms and bonds we can define. The octet rule says that in many compounds. Why Do Atoms Need 8 Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Why Do Atoms Need 8 Valence Electrons The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Molecules,. Why Do Atoms Need 8 Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Why Do Atoms Need 8 Valence Electrons The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). However, there are three general exceptions to the octet rule: Molecules in which one or more atoms possess more than. This is a consequence of the fact that many compounds involve the s and p block electrons,. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
Ions and Ionic Compounds ppt download Why Do Atoms Need 8 Valence Electrons The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Molecules in which one or more atoms possess more than. The 8. Why Do Atoms Need 8 Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Why Do Atoms Need 8 Valence Electrons This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. Molecules, such as no, with an odd number of electrons; Using quantum physics and its models of atoms and bonds we can define. The octet rule says that in many compounds the most. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
Bonds, Chemical Bonds Chapter ppt download Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. Molecules in which one or more atoms possess more than. Molecules, such as no, with an odd number of electrons; However, there are three general exceptions to the octet rule: The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds. Why Do Atoms Need 8 Valence Electrons.
From www.slideserve.com
PPT Unit 3 Periodic Table and Atom Structure PowerPoint Presentation Why Do Atoms Need 8 Valence Electrons However, there are three general exceptions to the octet rule: The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. Molecules, such as no, with an odd number of electrons; Molecules in which one or more atoms possess more than.. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
Octet Rule & Ions Unit 1 Notes. ppt download Why Do Atoms Need 8 Valence Electrons The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Atoms generally do not need 8 electrons to. Why Do Atoms Need 8 Valence Electrons.
From sciencenotes.org
Octet Rule Definition, Examples, and Exceptions Why Do Atoms Need 8 Valence Electrons However, there are three general exceptions to the octet rule: The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). Atoms generally do not need 8 electrons to stabilize. Using quantum physics and its models of atoms and bonds we can define. The 8 valence electron rule. Why Do Atoms Need 8 Valence Electrons.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary Why Do Atoms Need 8 Valence Electrons The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. However, there are. Why Do Atoms Need 8 Valence Electrons.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together Why Do Atoms Need 8 Valence Electrons This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. Using quantum physics and its models of atoms and bonds we can define. Atoms generally do not need 8 electrons to stabilize. Molecules in which one or more atoms possess more than. The. Why Do Atoms Need 8 Valence Electrons.
From www.slideserve.com
PPT Covalent Bonding Review PowerPoint Presentation, free download Why Do Atoms Need 8 Valence Electrons The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. This is a consequence of the fact that many compounds involve the. Why Do Atoms Need 8 Valence Electrons.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose Why Do Atoms Need 8 Valence Electrons The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4 orbitals and can thus contain 8 electrons. The octet rule says. Why Do Atoms Need 8 Valence Electrons.
From www.youtube.com
Why do atoms want 8 valence electrons? YouTube Why Do Atoms Need 8 Valence Electrons Molecules, such as no, with an odd number of electrons; The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four. Why Do Atoms Need 8 Valence Electrons.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Octet rule Why Do Atoms Need 8 Valence Electrons The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. The octet rule says that in many compounds the most stable (correct) electron configuration is when there are 8 electrons (four filled orbitals). Molecules in which one or more atoms possess. Why Do Atoms Need 8 Valence Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Why Do Atoms Need 8 Valence Electrons Atoms generally do not need 8 electrons to stabilize. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. The octet. Why Do Atoms Need 8 Valence Electrons.
From www.youtube.com
Why do atoms need 8 electrons to be stable? YouTube Why Do Atoms Need 8 Valence Electrons The 8 valence electron rule is based on the observation that atoms of the main group elements tend to form compounds in which each atom has eight electrons in its. Molecules, such as no, with an odd number of electrons; This is a consequence of the fact that many compounds involve the s and p block electrons, which contribute 4. Why Do Atoms Need 8 Valence Electrons.
From slideplayer.com
WHY DO ATOMS REACT?. ppt download Why Do Atoms Need 8 Valence Electrons Molecules in which one or more atoms possess more than. The octet rule states that atoms tend to prefer having 8 electrons in their valence shell in order to possess an electronic configuration similar to the closest noble gas. Using quantum physics and its models of atoms and bonds we can define. This is a consequence of the fact that. Why Do Atoms Need 8 Valence Electrons.