Electrode Potential Chemistry . The position of equilibrium and therefore the electrode potential depends on factors such as: In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they.
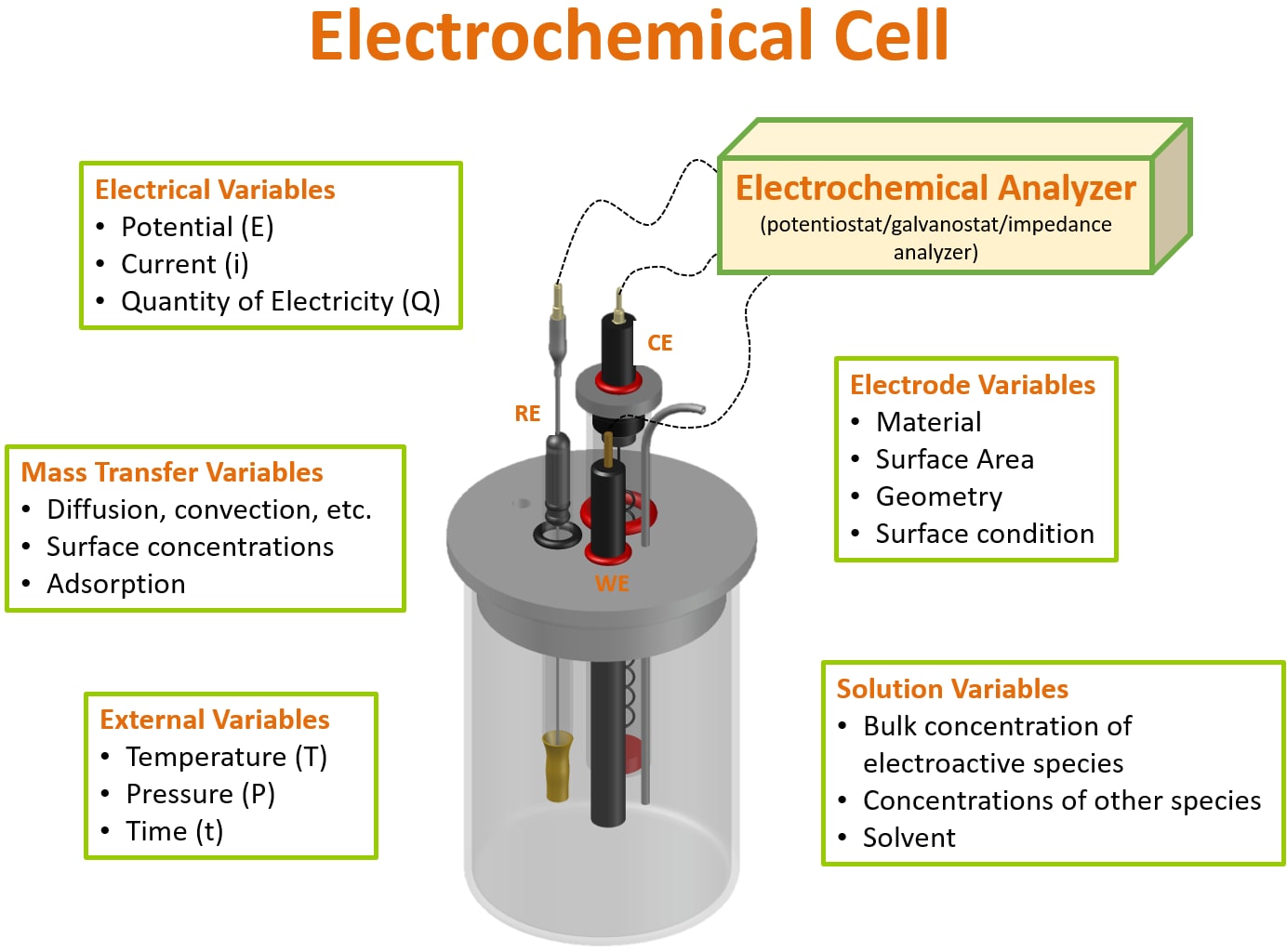
from www.sigmaaldrich.com
The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: An introduction to redox equilibria and electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. This page explains the background to standard electrode potentials (redox potentials), showing how they.
Electrochemistry on the Bench and in the Field
Electrode Potential Chemistry The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. This page explains the background to standard electrode potentials (redox potentials), showing how they. The position of equilibrium and therefore the electrode potential depends on factors such as: The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. An introduction to redox equilibria and electrode potentials. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Electrode Potential Chemistry The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The position of equilibrium and therefore the electrode potential depends on factors such as: An introduction to redox equilibria and. Electrode Potential Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry, standard electrode potential , or , is a measure of the. Electrode Potential Chemistry.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential Electrode Potential Chemistry In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. This page explains the background to standard electrode potentials (redox potentials), showing how they. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The position of equilibrium. Electrode Potential Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Potential Chemistry This page explains the background to standard electrode potentials (redox potentials), showing how they. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The oxidative and reductive. Electrode Potential Chemistry.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The position of equilibrium and therefore the electrode potential depends on factors such as: In electrochemistry, standard electrode. Electrode Potential Chemistry.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Electrode Potential Chemistry This page explains the background to standard electrode potentials (redox potentials), showing how they. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The oxidative and reductive. Electrode Potential Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrode Potential Chemistry The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. The position of equilibrium and therefore the electrode potential depends on factors such as: In electrochemistry, standard electrode potential , or , is a measure of the reducing power. Electrode Potential Chemistry.
From www.youtube.com
12th Chemistry Ch8Part7Trends in the M2+/M standard electrode Electrode Potential Chemistry In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The. Electrode Potential Chemistry.
From www.tes.com
AQA ALevel Chemistry Electrode Potentials and Electrochemical Cells Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The oxidative and reductive. Electrode Potential Chemistry.
From app.pandai.org
Standard Electrode Potential Electrode Potential Chemistry The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. This. Electrode Potential Chemistry.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry,. Electrode Potential Chemistry.
From www.youtube.com
Measurement of Electrode Potential, Chemistry Lecture Sabaq.pk YouTube Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: An introduction to redox equilibria and electrode potentials. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the. Electrode Potential Chemistry.
From classnotes.org.in
Electrochemical Series Chemistry, Class 12, Electro Chemistry Electrode Potential Chemistry The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The standard cell potential for a redox. Electrode Potential Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Potential Chemistry The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. An. Electrode Potential Chemistry.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. An introduction to redox. Electrode Potential Chemistry.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: This page explains the background to standard electrode potentials (redox potentials), showing how they. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The potential of an electrochemical cell is the difference between the potential. Electrode Potential Chemistry.
From chem.libretexts.org
20.2 Standard Electrode Potentials Chemistry LibreTexts Electrode Potential Chemistry This page explains the background to standard electrode potentials (redox potentials), showing how they. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The oxidative and reductive strengths of. Electrode Potential Chemistry.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Electrode Potential Chemistry The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The position of equilibrium and therefore the electrode potential depends on factors such as: An introduction to redox equilibria and electrode potentials. This. Electrode Potential Chemistry.
From www.shutterstock.com
Electrode Potentials Standard Hydrogen Electrode Standard Stock Vector Electrode Potential Chemistry In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox. Electrode Potential Chemistry.
From www.showme.com
AP 9.4a Electrode Potentials B Redox Reactions, Chemistry ShowMe Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The. Electrode Potential Chemistry.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 Electrode Potential Chemistry In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants. Electrode Potential Chemistry.
From chemistnotes.com
Single electrode potential Definition, origin, expression Chemistry Electrode Potential Chemistry The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. An introduction to redox equilibria and electrode potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. In electrochemistry, standard electrode potential , or , is a measure of the reducing power. Electrode Potential Chemistry.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Electrode Potential Chemistry The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants. Electrode Potential Chemistry.
From www.flexiprep.com
Electrochemistry Electrode Potential and Standard Electrode Potential Electrode Potential Chemistry The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element. Electrode Potential Chemistry.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Electrode Potential Chemistry In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The position of equilibrium and therefore the electrode potential depends on factors such as: The oxidative and reductive. Electrode Potential Chemistry.
From www.nagwa.com
Question Video Calculating Electrode Potential When Given a Cell Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The potential of an. Electrode Potential Chemistry.
From thechemistrynotes.com
Measuring the Standard Electrode Potential (Eꝋ) Electrode Potential Chemistry The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. An introduction to redox equilibria and electrode potentials. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such. Electrode Potential Chemistry.
From www.youtube.com
CHEMISTRY A LEVEL ELECTRODE POTENTIAL YouTube Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. This page explains the background to standard electrode potentials. Electrode Potential Chemistry.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Electrode Potential Chemistry The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox. Electrode Potential Chemistry.
From www.comsol.it
Approaching an Electrochemical Model from Scratch Lemon Battery Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the. Electrode Potential Chemistry.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode Electrode Potential Chemistry This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. In electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The. Electrode Potential Chemistry.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the potential at the anode,. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode. Electrode Potential Chemistry.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Electrode Potential Chemistry The position of equilibrium and therefore the electrode potential depends on factors such as: The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. This page explains the background to standard electrode potentials. Electrode Potential Chemistry.
From saylordotorg.github.io
Standard Potentials Electrode Potential Chemistry The oxidative and reductive strengths of a variety of substances can be compared using standard electrode potentials. An introduction to redox equilibria and electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: This page explains the background to standard electrode potentials (redox potentials), showing how they. The standard cell potential for a redox. Electrode Potential Chemistry.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Electrode Potential Chemistry An introduction to redox equilibria and electrode potentials. The position of equilibrium and therefore the electrode potential depends on factors such as: The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. The potential of an electrochemical cell is the difference between the potential at the cathode, \(e_\text{cathode}\), and the. Electrode Potential Chemistry.