Cs And Cl Form . caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. This colorless solid is an important source. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other.
from www.pikpng.com
This colorless solid is an important source. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole.
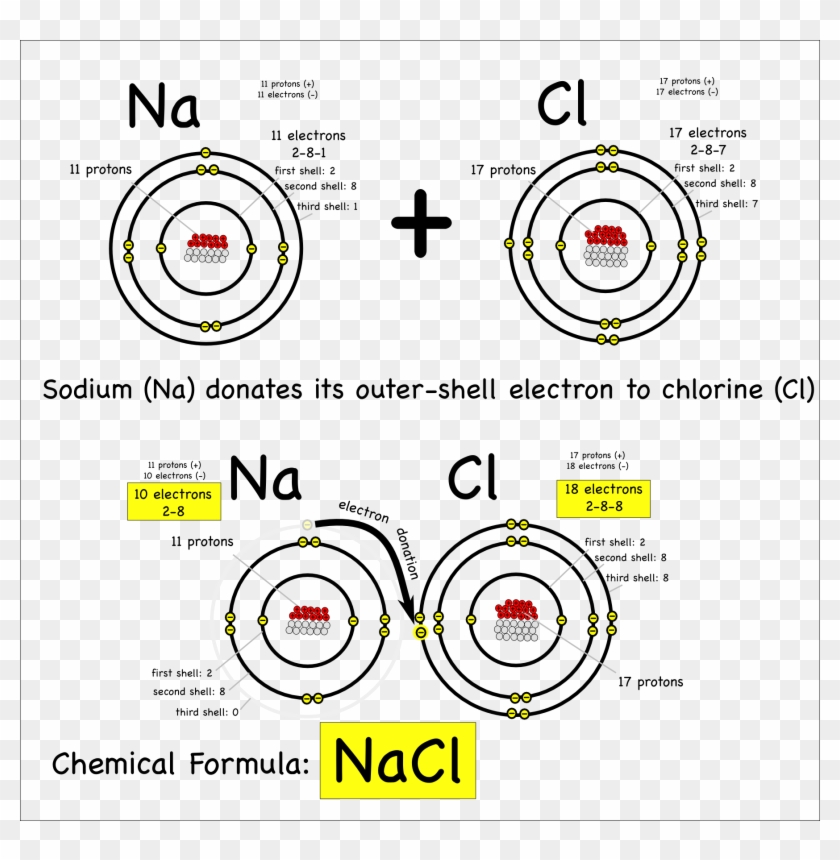
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of
Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. caesium chloride is the chemical compound with the formula cscl. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. This colorless solid is an important source. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol.
From www.chegg.com
Solved Caesium chloride (CsCl), which crystallizes in the Cs And Cl Form caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium. Cs And Cl Form.
From www.pdffiller.com
Unum Accident Claim Form Pdf Fill Online, Printable, Fillable, Blank Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. This colorless solid. Cs And Cl Form.
From byjus.com
In CsCl type structure, the coordination number of Cs+ and Cl Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with. Cs And Cl Form.
From commons.wikimedia.org
FileCalcium chloride CaCl2.jpg Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. This colorless solid is an important source. caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is. Cs And Cl Form.
From www.bartleby.com
Answered Cl Cs Cl Na S Zn Motif + Crystal… bartleby Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. caesium chloride is the chemical compound with the formula. Cs And Cl Form.
From exopqejyx.blob.core.windows.net
Chlorine Electron Pairs at Lewis Stallings blog Cs And Cl Form This colorless solid is an important source. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium chloride is the chemical compound with the formula cscl. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell. Cs And Cl Form.
From www.researchgate.net
SEMEDS elemental mapping of the polymer waste forms for C, S, Cs, Co Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. caesium + dichlorine = cesium chloride. caesium chloride is the chemical compound with the formula cscl. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. Cs And Cl Form.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Cs And Cl Form caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. This colorless solid is an important source. caesium. Cs And Cl Form.
From printableformsfree.com
Printable Unum Disability Forms Printable Forms Free Online Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. caesium + dichlorine = cesium chloride. caesium chloride is the chemical compound with the formula cscl. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of. Cs And Cl Form.
From www.slideserve.com
PPT Sodium Chloride Structure Na + Cl Cesium Chloride Structure C s Cs And Cl Form caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. This colorless solid is an important source. caesium chloride is the chemical compound with the formula cscl. cscl can be thought of as two interpenetrating simple cubic. Cs And Cl Form.
From brainly.in
In a cscl structure if edge length is x then distance between cs and cl Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium + dichlorine = cesium chloride. cscl can be thought of as two interpenetrating simple cubic. Cs And Cl Form.
From www.toppr.com
Cesium chloride is formed according to the following equation . Cs(s Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell. Cs And Cl Form.
From www.alamy.com
Sodium chloride. Computer graphic of crystallised common salt (sodium Cs And Cl Form caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium chloride is the chemical compound with the formula cscl.. Cs And Cl Form.
From www.youtube.com
CaCl2 Lewis Structure How to draw the Lewis Dot Structure for Calcium Cs And Cl Form cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is going to discuss the structure of. Cs And Cl Form.
From byjus.com
Select the incorrect statement in a CsCl crystal(a) Cs forms a simple Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. This colorless solid is an important source. caesium + dichlorine = cesium chloride. this page is going to discuss. Cs And Cl Form.
From www.pdffiller.com
20122024 Form Unum CL1019 Fill Online, Printable, Fillable, Blank Cs And Cl Form caesium + dichlorine = cesium chloride. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36. Cs And Cl Form.
From www.slideserve.com
PPT The Crystalline Solid State PowerPoint Presentation ID233957 Cs And Cl Form caesium chloride is the chemical compound with the formula cscl. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole.. Cs And Cl Form.
From formspal.com
Unum Beneficiary Designation PDF Form FormsPal Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. This colorless solid is an important source. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the. Cs And Cl Form.
From www.shutterstock.com
Crystal Lattice Cs Cl Cesium Chloride 库存插图 347363222 Shutterstock Cs And Cl Form This colorless solid is an important source. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page is going to discuss the structure. Cs And Cl Form.
From www.researchgate.net
Correlation between compressive strength(CS), chloride ion Cs And Cl Form This colorless solid is an important source. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. caesium +. Cs And Cl Form.
From alchetron.com
Caesium chloride Alchetron, The Free Social Encyclopedia Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium chloride is the chemical compound with the formula cscl. This colorless solid is an important source. this page is going to discuss the structure of the molecule cesium chloride. Cs And Cl Form.
From mavink.com
Cscl Unit Cell Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium chloride is the chemical compound with the formula cscl. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the. Cs And Cl Form.
From www.researchgate.net
Plot of Cl versus B, Cl versus F, Cl versus SiO2, Cl versus Cs, Cl Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium + dichlorine = cesium chloride. . Cs And Cl Form.
From study.com
Calcium Chloride Formula, Compound Name & Structure Lesson Cs And Cl Form caesium chloride is the chemical compound with the formula cscl. caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. Cs And Cl Form.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Cs And Cl Form caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. This. Cs And Cl Form.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium + dichlorine = cesium chloride. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page. Cs And Cl Form.
From www.researchgate.net
The illustration of CL and Cs Download Scientific Diagram Cs And Cl Form cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. caesium chloride is the chemical compound with the formula cscl. caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and. Cs And Cl Form.
From fillableforms.net
Dfas Form 9098 PDF Fillable Fillable Form 2024 Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. This colorless solid is an important source. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. caesium + dichlorine = cesium chloride. caesium chloride is. Cs And Cl Form.
From www.researchgate.net
Sketches of the possible arrangements of the CsCl structure type Cs And Cl Form This colorless solid is an important source. caesium + dichlorine = cesium chloride. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol.. Cs And Cl Form.
From www.youtube.com
CSGO how to Bind the cl_righthand 0 command and toggle it ENG YouTube Cs And Cl Form caesium chloride is the chemical compound with the formula cscl. This colorless solid is an important source. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium. Cs And Cl Form.
From www.scribd.com
6 Cs CL PDF Computer Network Domain Name Cs And Cl Form This colorless solid is an important source. caesium chloride is the chemical compound with the formula cscl. cscl can be thought of as two interpenetrating simple cubic arrays where the corner of one cell sits at the body center of the other. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl),. Cs And Cl Form.
From exobbjxou.blob.core.windows.net
Chlorine Oxide Chemical Formula at Shirley Becnel blog Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a mass of 168.36 g/mol. caesium + dichlorine = cesium chloride. This. Cs And Cl Form.
From www.youtube.com
Lewis Structure of CsCl, caesium chloride YouTube Cs And Cl Form This colorless solid is an important source. caesium + dichlorine = cesium chloride. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. cscl can be. Cs And Cl Form.
From docslib.org
289. Problem 22.35P (HRW) in the Basic Cscl (Caesium Chloride) Crystal Cs And Cl Form this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. caesium chloride is the chemical compound with the formula cscl. This colorless solid is an important source. Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole.. Cs And Cl Form.
From www.doubtnut.com
In a CsCl structure, if edge length is a, then distance between one Cs Cs And Cl Form Cs + cl2 = cscl is a synthesis reaction where two moles of caesium [cs] and one mole. this page is going to discuss the structure of the molecule cesium chloride (cscl cscl), which is a white hydroscopic solid with a. This colorless solid is an important source. caesium chloride is the chemical compound with the formula cscl.. Cs And Cl Form.