Bromine Atom Oxidation State . the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. It is defined as being the charge that an atom. the oxidation number is also known as the oxidation state. the most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. the oxidation state of the central bromine atom will be: bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. In neutral compounds, the sum state of all the atoms is zero. the oxidation state of an atom is a measure of the degree of oxidation of an atom.
from www.chegg.com
bromine's oxidation numbers. In neutral compounds, the sum state of all the atoms is zero. the oxidation state of the central bromine atom will be: the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation number is also known as the oxidation state. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. It is defined as being the charge that an atom. But oxidation states of 0 (elemental bromine, br 2),. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation state of an atom is a measure of the degree of oxidation of an atom.
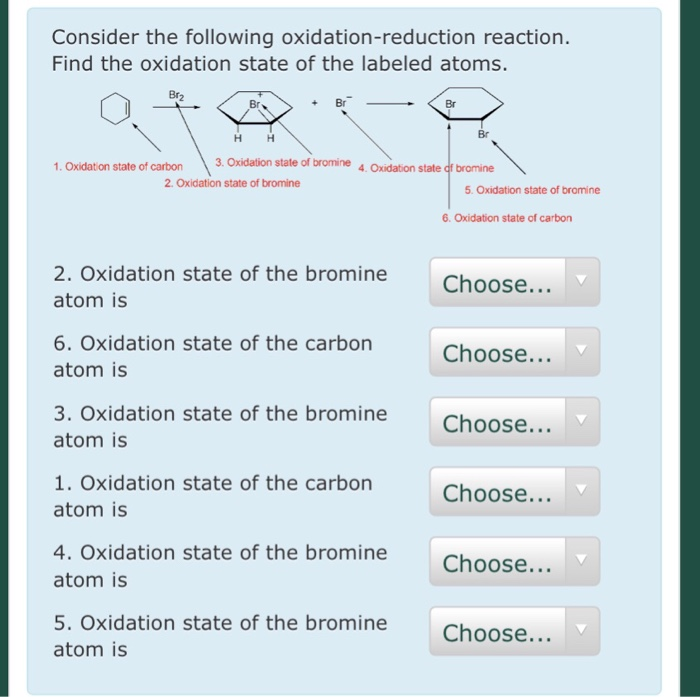
Solved Consider the following oxidationreduction reaction.
Bromine Atom Oxidation State bromine's oxidation numbers. But oxidation states of 0 (elemental bromine, br 2),. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation state. It is defined as being the charge that an atom. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation state of an atom is a measure of the degree of oxidation of an atom. In neutral compounds, the sum state of all the atoms is zero. the oxidation state of the central bromine atom will be: bromine's oxidation numbers.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Atom Oxidation State bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation state of an atom is a measure of the degree of oxidation of an atom. But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. the most stable oxidation state of the element is −1,. Bromine Atom Oxidation State.
From www.numerade.com
Determine the oxidation state for each of the elements below. The Bromine Atom Oxidation State bromine's oxidation numbers. In neutral compounds, the sum state of all the atoms is zero. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation state.. Bromine Atom Oxidation State.
From www.numerade.com
SOLVED Why is HBrO the weakest acid when compared to HBrO2, HBrO3 and Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation state. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. It is defined as being the charge that an atom. But oxidation states of. Bromine Atom Oxidation State.
From byjus.com
What is the oxidation number of bromine in sequence in Br3O8? Bromine Atom Oxidation State In neutral compounds, the sum state of all the atoms is zero. the oxidation state of the central bromine atom will be: But oxidation states of 0 (elemental bromine, br 2),. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state of an atom is a measure of the. Bromine Atom Oxidation State.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Atom Oxidation State the oxidation state of an atom is a measure of the degree of oxidation of an atom. bromine's oxidation numbers. the oxidation state of the central bromine atom will be: the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation. Bromine Atom Oxidation State.
From askfilo.com
Consider the change in oxidation state of Bromine corresponding to differ.. Bromine Atom Oxidation State the oxidation number is also known as the oxidation state. In neutral compounds, the sum state of all the atoms is zero. bromine's oxidation numbers. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. bromine is a chemical element with atomic number 35 which means there are 35 protons. Bromine Atom Oxidation State.
From askfilo.com
Consider the change in oxidation state of Bromine corresponding to differ.. Bromine Atom Oxidation State It is defined as being the charge that an atom. In neutral compounds, the sum state of all the atoms is zero. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation. Bromine Atom Oxidation State.
From www.numerade.com
SOLVED The unbalanced equation for the reaction of sulfur dioxide with Bromine Atom Oxidation State the oxidation state of the central bromine atom will be: But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. the most stable oxidation state of the element is −1, in which bromine occurs naturally. It is defined as being the charge that an atom. the oxidation number is also known as the oxidation. Bromine Atom Oxidation State.
From edurev.in
Oxidation state of Central Br atom in Br3O8 is? EduRev NEET Question Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. It is defined as being the charge that an atom. the most stable oxidation state of the element is −1, in which bromine occurs naturally. bromine's oxidation numbers. But oxidation states of 0 (elemental bromine, br 2),. In neutral compounds, the. Bromine Atom Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Atom Oxidation State the most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. the oxidation state of the central bromine atom will be: bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the. Bromine Atom Oxidation State.
From www.chegg.com
Solved Calculate the oxidation state of the bromine atom in Bromine Atom Oxidation State the most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2),. In neutral compounds, the sum state of all the atoms is zero. the oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state tells. Bromine Atom Oxidation State.
From www.chegg.com
Solved whats is tje oxidation state of an individual bromine Bromine Atom Oxidation State bromine's oxidation numbers. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state of. Bromine Atom Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Atom Oxidation State the oxidation number is also known as the oxidation state. But oxidation states of 0 (elemental bromine, br 2),. the oxidation state of the central bromine atom will be: the oxidation state of an atom is a measure of the degree of oxidation of an atom. bromine is a chemical element with atomic number 35 which. Bromine Atom Oxidation State.
From www.chegg.com
Solved What is the change in oxidation state for the bromine Bromine Atom Oxidation State the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation number is also known as the oxidation state. bromine's oxidation numbers. the oxidation state of the central bromine atom will be: It is defined as being the charge that an atom. bromine is a chemical element with atomic. Bromine Atom Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. bromine's oxidation numbers. It is defined as being the charge that an atom. In neutral compounds, the sum state of all the atoms is zero. the oxidation number is also known as the oxidation state. bromine is a chemical element. Bromine Atom Oxidation State.
From askfilo.com
(15.) Consider the change in oxidation state of bromine corresponding to Bromine Atom Oxidation State bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. But oxidation states of 0 (elemental bromine, br 2),. the oxidation state of the central bromine atom will be: the oxidation number is. Bromine Atom Oxidation State.
From byjus.com
to find oxidation no of central bromine atom in Br_3O_ Bromine Atom Oxidation State bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation state of the central bromine atom will be: But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. . Bromine Atom Oxidation State.
From www.chegg.com
Solved Consider the following oxidationreduction reaction. Bromine Atom Oxidation State In neutral compounds, the sum state of all the atoms is zero. bromine's oxidation numbers. It is defined as being the charge that an atom. the oxidation state of the central bromine atom will be: bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the most stable oxidation. Bromine Atom Oxidation State.
From byjus.com
Oxidation state of oxygen in Tribromo Octaoxide (Br3O8) is Options 16/ Bromine Atom Oxidation State bromine's oxidation numbers. In neutral compounds, the sum state of all the atoms is zero. But oxidation states of 0 (elemental bromine, br 2),. the oxidation number is also known as the oxidation state. the oxidation state of the central bromine atom will be: It is defined as being the charge that an atom. the oxidation. Bromine Atom Oxidation State.
From www.numerade.com
SOLVED Determine the oxidation state of each bromine atom Co(BrO2)2 Bromine Atom Oxidation State But oxidation states of 0 (elemental bromine, br 2),. In neutral compounds, the sum state of all the atoms is zero. bromine's oxidation numbers. It is defined as being the charge that an atom. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation number is also known. Bromine Atom Oxidation State.
From hillruslaideemin.blogspot.com
How Do You Find The Charge Of An Atom Hill Ruslaideemin Bromine Atom Oxidation State It is defined as being the charge that an atom. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2),. bromine is a chemical element with atomic. Bromine Atom Oxidation State.
From www.toppr.com
Consider the change in oxidation state of Bromine corresponding to Bromine Atom Oxidation State bromine's oxidation numbers. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation number. Bromine Atom Oxidation State.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Oxidation State But oxidation states of 0 (elemental bromine, br 2),. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the most stable oxidation state of the element is −1, in which bromine occurs naturally.. Bromine Atom Oxidation State.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation state. In neutral compounds, the sum state of all the atoms is zero. But oxidation states of 0 (elemental bromine, br 2),. It is defined as being the charge that an atom. . Bromine Atom Oxidation State.
From quizlet.com
What is the oxidation number of \ce{Br} in \ce{Br3O8}? Quizlet Bromine Atom Oxidation State bromine's oxidation numbers. the oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that an atom. But oxidation states of 0 (elemental bromine, br 2),. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the. Bromine Atom Oxidation State.
From ar.inspiredpencil.com
Hobr Lewis Structure Bromine Atom Oxidation State But oxidation states of 0 (elemental bromine, br 2),. the oxidation number is also known as the oxidation state. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. bromine's oxidation numbers. the oxidation state of an atom is a measure of the degree of oxidation of an atom. . Bromine Atom Oxidation State.
From www.compoundchem.com
The Periodic Table of Oxidation States Compound Interest Bromine Atom Oxidation State bromine is a chemical element with atomic number 35 which means there are 35 protons and 35. the oxidation number is also known as the oxidation state. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation state of the central bromine atom will be: the oxidation state. Bromine Atom Oxidation State.
From www.youtube.com
Consider the change in oxidation state of bromine corresponding to Bromine Atom Oxidation State the oxidation number is also known as the oxidation state. But oxidation states of 0 (elemental bromine, br 2),. bromine's oxidation numbers. It is defined as being the charge that an atom. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. In neutral compounds, the sum state of all the. Bromine Atom Oxidation State.
From www.chegg.com
Solved During bromination reaction of Estilbene with Bromine Atom Oxidation State the oxidation state of an atom is a measure of the degree of oxidation of an atom. It is defined as being the charge that an atom. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation state of the central bromine atom will be: In neutral compounds, the. Bromine Atom Oxidation State.
From www.doubtnut.com
Consider the change in oxidation state of Bromine corredponding to dif Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the oxidation number is also known as the oxidation state. the oxidation state of the central bromine atom will be: But oxidation states of 0 (elemental bromine, br 2),. It is defined as being the charge that an atom. In neutral. Bromine Atom Oxidation State.
From www.youtube.com
What is the oxidation state of an individual bromine atom in ? YouTube Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. In neutral compounds, the sum state of all the atoms is zero. the oxidation number is also known as the oxidation state. the oxidation state of an atom is a measure of the degree of oxidation of an atom. the. Bromine Atom Oxidation State.
From www.youtube.com
How to find the Oxidation Number for Br in Br2 (Bromine gas) YouTube Bromine Atom Oxidation State In neutral compounds, the sum state of all the atoms is zero. the oxidation state of an atom is a measure of the degree of oxidation of an atom. bromine's oxidation numbers. But oxidation states of 0 (elemental bromine, br 2),. the oxidation state of the central bromine atom will be: the most stable oxidation state. Bromine Atom Oxidation State.
From www.numerade.com
SOLVED 'Some chemical reactants are listed in the table below Bromine Atom Oxidation State the oxidation state of an atom is a measure of the degree of oxidation of an atom. the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. In neutral compounds, the sum state of all the atoms is zero. the oxidation number is also known as the oxidation state. But oxidation. Bromine Atom Oxidation State.
From www.youtube.com
How to calculate Oxidation number of Br in Br3O8? YouTube Bromine Atom Oxidation State the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation number is also known as the oxidation state. the oxidation state of an atom is a measure of the degree of oxidation of an atom. bromine's oxidation numbers. In neutral compounds, the sum state of all the atoms is. Bromine Atom Oxidation State.
From giohgponx.blob.core.windows.net
Zinc And Oxidation State at Hester Cooper blog Bromine Atom Oxidation State the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. the most stable oxidation state of the element is −1, in which bromine occurs naturally. the oxidation number is also known as the oxidation state. It is defined as being the charge that an atom. But oxidation states of 0 (elemental. Bromine Atom Oxidation State.