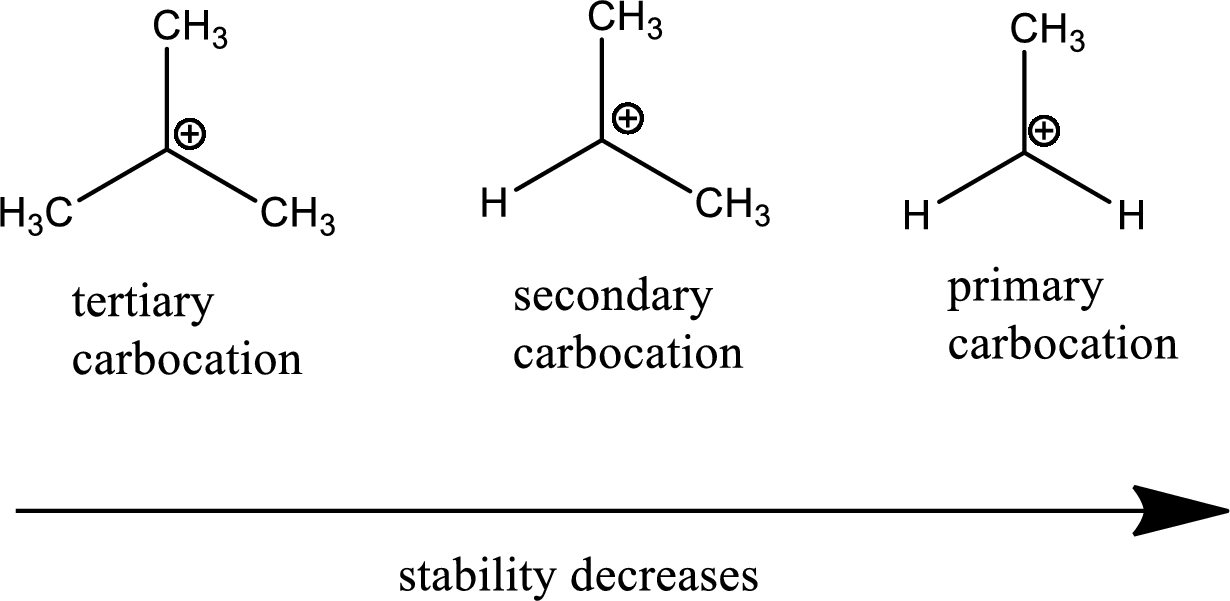
Stable Carbocations . three main factors increase the stability of carbocations: Increasing the number of adjacent carbon atoms: To understand why markovnikov’s rule works, we need to. carbocation structure and stability. describe the geometry of a given carbocation. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. welcome to today’s deep dive into the world of carbocations. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability.
from www.bartleby.com
welcome to today’s deep dive into the world of carbocations. describe the geometry of a given carbocation. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. carbocation structure and stability. Increasing the number of adjacent carbon atoms: one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. To understand why markovnikov’s rule works, we need to. three main factors increase the stability of carbocations: Arrange a given series of carbocations in order of increasing or decreasing stability. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation.
Arrange these carbocations in order of increasing stability. (a) (b) (c
Stable Carbocations Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. carbocation structure and stability. To understand why markovnikov’s rule works, we need to. Increasing the number of adjacent carbon atoms: three main factors increase the stability of carbocations: welcome to today’s deep dive into the world of carbocations. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. describe the geometry of a given carbocation. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability.
From www.chegg.com
Solved Which carbocation is the most stable? B & C are Stable Carbocations one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. To understand why markovnikov’s rule works, we need to. carbocation structure and stability. welcome to today’s deep dive into the world of carbocations. Arrange a given series of carbocations in order of increasing or decreasing stability. describe. Stable Carbocations.
From www.masterorganicchemistry.com
Rearrangement Reactions (1) Hydride Shifts Master Organic Chemistry Stable Carbocations carbocation structure and stability. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. Increasing. Stable Carbocations.
From www.aceorganicchem.com
Carbocation Stability [with free study guide] organic chemistry help Stable Carbocations three main factors increase the stability of carbocations: Increasing the number of adjacent carbon atoms: carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. welcome to today’s deep dive into the world of carbocations. describe the geometry of a given carbocation. carbocation structure and stability. one way of. Stable Carbocations.
From www.coursehero.com
[Solved] . Why are 3 carbocations so much more stable than 29, 1", or Stable Carbocations three main factors increase the stability of carbocations: Arrange a given series of carbocations in order of increasing or decreasing stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. describe the geometry of a given carbocation. carbocation stability is crucial because it determines the rate and mechanism. Stable Carbocations.
From www.numerade.com
SOLVED For each of the groups of compounds shown below; rank the Stable Carbocations To understand why markovnikov’s rule works, we need to. Increasing the number of adjacent carbon atoms: describe the geometry of a given carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. three main factors increase the stability of carbocations: carbocation stability is crucial because it. Stable Carbocations.
From www.toppr.com
Which of the following is most stable carbocations Stable Carbocations Arrange a given series of carbocations in order of increasing or decreasing stability. describe the geometry of a given carbocation. carbocation structure and stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. To understand why markovnikov’s rule works, we need to. Increasing the number of adjacent carbon atoms:. Stable Carbocations.
From www.numerade.com
Identify the most stable carbocation. Stable Carbocations welcome to today’s deep dive into the world of carbocations. describe the geometry of a given carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. Increasing the number of adjacent carbon atoms: three main factors increase the stability of carbocations: To understand why markovnikov’s rule. Stable Carbocations.
From www.transtutors.com
(Get Answer) 2Rank the following carbocations in order of increasing Stable Carbocations three main factors increase the stability of carbocations: welcome to today’s deep dive into the world of carbocations. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations. Stable Carbocations.
From www.chegg.com
Solved Rank the carbocations shown below from least stable Stable Carbocations describe the geometry of a given carbocation. welcome to today’s deep dive into the world of carbocations. Arrange a given series of carbocations in order of increasing or decreasing stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. carbocation structure and stability. three main factors increase. Stable Carbocations.
From www.chegg.com
Solved Which of the following carbocations is least stable? Stable Carbocations carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. three main factors increase the stability of carbocations: describe the geometry of a given carbocation. carbocation structure and stability. Arrange a given series of carbocations in order of increasing or decreasing stability. Increasing the number of adjacent carbon atoms: one. Stable Carbocations.
From www.chegg.com
Solved Rank the following carbocations from least stable to Stable Carbocations Increasing the number of adjacent carbon atoms: Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. three main factors increase the stability of carbocations: By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Arrange a given. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations carbocation structure and stability. welcome to today’s deep dive into the world of carbocations. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. By the end of this tutorial, you’ll grasp the intricacies. Stable Carbocations.
From askfilo.com
Intermediate (Major) The most stable carbocation is Filo Stable Carbocations three main factors increase the stability of carbocations: carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. carbocation structure and stability. Increasing the number of adjacent carbon atoms: Explain the relative. Stable Carbocations.
From socratic.org
What stabilizes carbocation? Socratic Stable Carbocations Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. To understand why markovnikov’s rule works, we need to. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. carbocation structure and stability. By the end of this tutorial, you’ll grasp. Stable Carbocations.
From brainly.in
which of the following carbocation is most stable Brainly.in Stable Carbocations carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Increasing the number of adjacent carbon atoms: Arrange a given series of carbocations in order of increasing or decreasing stability. three main factors increase the stability of carbocations: By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. carbocation structure and stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. one way of determining carbocation stabilities is to measure the amount of energy. Stable Carbocations.
From nathanielghopzimmerman.blogspot.com
1 Which of the Following Is the Most Stable Carbocation Stable Carbocations describe the geometry of a given carbocation. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. carbocation structure and stability. Arrange a given series of carbocations in order of increasing or decreasing stability. welcome to today’s deep dive into the world of carbocations. one way of determining carbocation stabilities. Stable Carbocations.
From www.toppr.com
Which one of the following carbocations is most stable Stable Carbocations Increasing the number of adjacent carbon atoms: welcome to today’s deep dive into the world of carbocations. Arrange a given series of carbocations in order of increasing or decreasing stability. To understand why markovnikov’s rule works, we need to. By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries. Stable Carbocations.
From askfilo.com
43. The most stable carbocation is (1) CH3 −CH2 ⊕ Filo Stable Carbocations carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. describe the geometry of a given carbocation. carbocation structure and stability. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. By the end of this tutorial, you’ll grasp the intricacies of their structure,. Stable Carbocations.
From www.bartleby.com
Arrange these carbocations in order of increasing stability. (a) (b) (c Stable Carbocations describe the geometry of a given carbocation. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Arrange a given series of carbocations in order of increasing or decreasing stability.. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. describe the geometry of a given carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability. . Stable Carbocations.
From xecogioinhapkhau.com
Why Does Carbon Stable Unveiling Its Atomic Secrets Stable Carbocations Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Increasing the number of adjacent carbon atoms: Arrange a given series of carbocations in order of increasing or decreasing stability. three main factors increase the. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Arrange a given series of carbocations in order of increasing or decreasing stability. To understand why markovnikov’s rule works, we need to. three main factors increase the stability of carbocations: one way of determining carbocation. Stable Carbocations.
From www.coursehero.com
[Solved] 1. Rank the following carbocations from most to least stable Stable Carbocations Increasing the number of adjacent carbon atoms: By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. To understand why markovnikov’s rule works, we need to. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. . Stable Carbocations.
From www.toppr.com
34. Which carbocation is stable CH3 CH2 CH) CH3 CD3 Stable Carbocations carbocation structure and stability. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. three main factors increase the stability of carbocations: By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. carbocation stability. Stable Carbocations.
From www.numerade.com
SOLVED Draw a structural formula for the more stable carbocation Stable Carbocations To understand why markovnikov’s rule works, we need to. Arrange a given series of carbocations in order of increasing or decreasing stability. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. welcome. Stable Carbocations.
From byjus.com
Is phenyl carbanion and carbocation stable? Are they both aromatic? Are Stable Carbocations By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Increasing the number of adjacent carbon atoms: Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. To understand why markovnikov’s rule works, we need to. describe the. Stable Carbocations.
From www.toppr.com
Most stable carbocation is Chemistry Questions Stable Carbocations carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. carbocation structure and stability. three main factors increase the stability of carbocations: describe the geometry of a given carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability. To understand why markovnikov’s rule works, we need to.. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations welcome to today’s deep dive into the world of carbocations. Increasing the number of adjacent carbon atoms: three main factors increase the stability of carbocations: Explain the relative stability of methyl, primary, secondary and tertiary carbocations in terms of hyperconjugation and inductive effects. describe the geometry of a given carbocation. Arrange a given series of carbocations in. Stable Carbocations.
From www.coursehero.com
[Solved] order these carbocations from most stable to least stable. If Stable Carbocations welcome to today’s deep dive into the world of carbocations. Arrange a given series of carbocations in order of increasing or decreasing stability. describe the geometry of a given carbocation. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. three main factors increase the stability of. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations three main factors increase the stability of carbocations: one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. Arrange a given series of carbocations in order of increasing or decreasing stability. welcome to today’s deep dive into the world of carbocations. To understand why markovnikov’s rule works, we. Stable Carbocations.
From chemistry2134.blogspot.com
Carbocations Factors affecting their Stability Stable Carbocations welcome to today’s deep dive into the world of carbocations. carbocation structure and stability. Arrange a given series of carbocations in order of increasing or decreasing stability. three main factors increase the stability of carbocations: describe the geometry of a given carbocation. one way of determining carbocation stabilities is to measure the amount of energy. Stable Carbocations.
From www.askiitians.com
Which of the following is the most stable carbocation ? askIITians Stable Carbocations By the end of this tutorial, you’ll grasp the intricacies of their structure, understand their stability, and unravel the mysteries of carbocation rearrangements. Increasing the number of adjacent carbon atoms: one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. welcome to today’s deep dive into the world of. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation. Arrange a given series of carbocations in order of increasing or decreasing stability. carbocation structure and stability. welcome to today’s deep dive. Stable Carbocations.
From www.masterorganicchemistry.com
3 Factors That Stabilize Carbocations Stable Carbocations welcome to today’s deep dive into the world of carbocations. one way of determining carbocation stabilities is to measure the amount of energy required to form a carbocation by. To understand why markovnikov’s rule works, we need to. describe the geometry of a given carbocation. carbocation stability is crucial because it determines the rate and mechanism. Stable Carbocations.