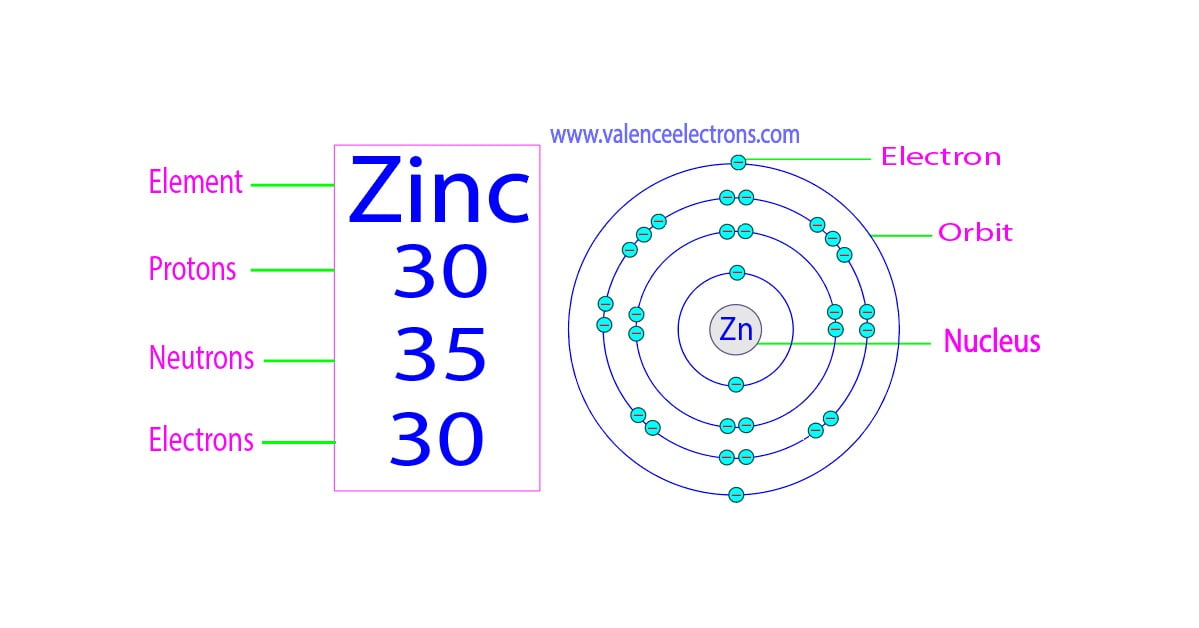
Zinc Have A Negative Charge . As zinc is a metal, it generally forms metallic compounds with. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. The average human body contains about. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. [1] you can see that the outermost orbit of zinc has 2 electrons. Zinc is one of the least common elements and is. the electron configuration of zinc is: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).
from valenceelectrons.com
if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. the electron configuration of zinc is: [1] you can see that the outermost orbit of zinc has 2 electrons. The average human body contains about. As zinc is a metal, it generally forms metallic compounds with. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. Zinc is one of the least common elements and is. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii.
How Many Protons, Neutrons and Electrons Does Zinc Have?
Zinc Have A Negative Charge zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. the electron configuration of zinc is: zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. The average human body contains about. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. Zinc is one of the least common elements and is. [1] you can see that the outermost orbit of zinc has 2 electrons. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. As zinc is a metal, it generally forms metallic compounds with.
From store.drjockers.com
Zinc Charge Dr. Jockers Store Zinc Have A Negative Charge The average human body contains about. the electron configuration of zinc is: As zinc is a metal, it generally forms metallic compounds with. Zinc is one of the least common elements and is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). zinc will rarely form ions. Zinc Have A Negative Charge.
From cerzmopv.blob.core.windows.net
Magnesium And Zinc Nitrate Equation at Terry Ranson blog Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. [1] you can see that the outermost orbit of zinc has 2 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). During the chemical reaction, zinc loses. Zinc Have A Negative Charge.
From www.shutterstock.com
266 Basic Electrical Theory Images, Stock Photos, 3D objects, & Vectors Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.. Zinc Have A Negative Charge.
From store.drjockers.com
Zinc Charge Dr. Jockers Store Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are. Zinc Have A Negative Charge.
From www.charge-transfer.pl
Properties of zinc coatings electrochemically passivated in sodium Zinc Have A Negative Charge Zinc is one of the least common elements and is. the electron configuration of zinc is: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. During. Zinc Have A Negative Charge.
From giontpeub.blob.core.windows.net
Does Positive Energy Attract Negative Energy at Terrence Rafael blog Zinc Have A Negative Charge if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1. Zinc Have A Negative Charge.
From cerzmopv.blob.core.windows.net
Magnesium And Zinc Nitrate Equation at Terry Ranson blog Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. the electron configuration of zinc is: zinc will rarely form ions with a +1 charge. Zinc Have A Negative Charge.
From www.mining.com
Column Zinc treatment charges jump after 2022 smelter bottleneck Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. the electron configuration of zinc is: The average human body contains about. Zinc is one of the least. Zinc Have A Negative Charge.
From www.alamy.com
Attract and repel positive and negative charge. Like charges repel and Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. the electron configuration of zinc is: zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. During the chemical reaction, zinc loses these 2 electrons and achieves the. Zinc Have A Negative Charge.
From byjus.com
What is a positive or negative charges? Zinc Have A Negative Charge zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. Zinc is one of the least common elements and is. if an atom, or atoms, has a balanced number of. Zinc Have A Negative Charge.
From www.researchgate.net
Galvanostatic chargedischarge cycles of zincair batteries. Download Zinc Have A Negative Charge [1] you can see that the outermost orbit of zinc has 2 electrons. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. The average human body contains about. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. Zinc Have A Negative Charge.
From solverpharma.com
ZincCharge Solver Agropharma Ltd. Zinc Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). As zinc is a metal, it generally forms metallic compounds with. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. 1s 2 2s 2 2p 6 3s 2. Zinc Have A Negative Charge.
From www.gd-huaren.net
Which is better, oxidation primer or zinc primer? Guangdong Huaren Zinc Have A Negative Charge As zinc is a metal, it generally forms metallic compounds with. Zinc is one of the least common elements and is. the electron configuration of zinc is: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). if an atom, or atoms, has a balanced number of electrons. Zinc Have A Negative Charge.
From www.mdpi.com
IJERPH Free FullText The Essential Toxin Impact of Zinc on Human Zinc Have A Negative Charge the electron configuration of zinc is: for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). As zinc is a metal, it generally forms metallic compounds with. [1]. Zinc Have A Negative Charge.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Zinc Have? Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. As zinc is a metal, it generally forms metallic compounds with. Zinc is one of the least common elements and is. [1] you can see that the outermost orbit of zinc has 2 electrons. During the chemical reaction, zinc. Zinc Have A Negative Charge.
From fyomgzclo.blob.core.windows.net
Why Do I Want To Throw Up After Taking Zinc at Jacob Dunbar blog Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. When the atom loses or gains one or more electrons, the electric charge is generated (and an. Zinc Have A Negative Charge.
From hxeoyhyjm.blob.core.windows.net
Zinc Refining Charge at Gina Doyle blog Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are. Zinc Have A Negative Charge.
From schematicploraireswsjc0.z13.web.core.windows.net
How To Draw An Electrochemical Cell Zinc Have A Negative Charge the electron configuration of zinc is: As zinc is a metal, it generally forms metallic compounds with. [1] you can see that the outermost orbit of zinc has 2 electrons. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. The average human body contains about. During the. Zinc Have A Negative Charge.
From sciencetrends.com
What Is The Ionic Charge Of Zinc (Zn)? Science Trends Zinc Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). As zinc is a metal, it generally forms metallic compounds with. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. the electron configuration of zinc is: 1s. Zinc Have A Negative Charge.
From cerzmopv.blob.core.windows.net
Magnesium And Zinc Nitrate Equation at Terry Ranson blog Zinc Have A Negative Charge zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. Zinc is one of the least common elements and is. When the atom loses or gains one or more electrons, the. Zinc Have A Negative Charge.
From fyowclttk.blob.core.windows.net
Zinc Solid Charge at Diane Garcia blog Zinc Have A Negative Charge [1] you can see that the outermost orbit of zinc has 2 electrons. the electron configuration of zinc is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group. Zinc Have A Negative Charge.
From socratic.org
How do you write the formula for zinc acetate? Socratic Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. The average human body contains about. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. for example, group i elements on the periodic table, known as. Zinc Have A Negative Charge.
From exowsidwq.blob.core.windows.net
Zinc How To Find Charge at Patel blog Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group. Zinc Have A Negative Charge.
From store.drjockers.com
Zinc Charge Dr. Jockers Store Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. The average human body contains about. As zinc is a metal, it generally forms metallic compounds with. [1] you can see that the outermost orbit of zinc has 2 electrons. if an atom, or atoms, has a balanced number of. Zinc Have A Negative Charge.
From www.alamy.com
Early battery, working by chemical reaction, with zinc strip as Zinc Have A Negative Charge for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. Zinc is one of the least common elements and is. As zinc is a metal, it generally forms metallic compounds with. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge). Zinc Have A Negative Charge.
From fyoiablom.blob.core.windows.net
Zinc Ion Identification at Paul Lombardi blog Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. the electron configuration of zinc is: zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. [1] you can see that the outermost orbit of zinc has 2 electrons. . Zinc Have A Negative Charge.
From www.britannica.com
Zinc Properties, Uses, & Facts Britannica Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). the electron configuration of zinc is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Zinc Have A Negative Charge.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight Zinc Have A Negative Charge As zinc is a metal, it generally forms metallic compounds with. Zinc is one of the least common elements and is. the electron configuration of zinc is: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). [1] you can see that the outermost orbit of zinc has 2. Zinc Have A Negative Charge.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Have A Negative Charge if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. the electron configuration of zinc is: As zinc is a metal, it generally forms metallic compounds with. The average human body contains about. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Zinc Have A Negative Charge.
From store.drjockers.com
Zinc Charge Dr. Jockers Store Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. zinc will rarely form ions with a +1 charge but it will never form ions with a. Zinc Have A Negative Charge.
From byjus.com
In a dry cell ,zinc container acts as terminal and carbon rod acts as Zinc Have A Negative Charge The average human body contains about. zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. for example, group i elements on the periodic table, known as alkali metals,. Zinc Have A Negative Charge.
From www.slideserve.com
PPT Types of Chemical Bonds PowerPoint Presentation, free download Zinc Have A Negative Charge When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The average human body contains about. As zinc is a metal, it generally forms metallic compounds with. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. [1] you can see. Zinc Have A Negative Charge.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode Zinc Have A Negative Charge As zinc is a metal, it generally forms metallic compounds with. During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. for example, group i elements on the periodic table,. Zinc Have A Negative Charge.
From www.kokilabenhospital.com
Signs of Zinc Deficiency Health Tips from Kokilaben Hospital Zinc Have A Negative Charge 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 or [ar] 3d 10 4s 2. for example, group i elements on the periodic table, known as alkali metals, typically possess a +1 charge, group ii. the electron configuration of zinc is: During the chemical reaction, zinc loses these 2 electrons and achieves the. Zinc Have A Negative Charge.
From academicsongs.com
What charge does zinc have? Academic Hacks Zinc Have A Negative Charge During the chemical reaction, zinc loses these 2 electrons and achieves the nearest noble gas configuration to become stable. if an atom, or atoms, has a balanced number of electrons (negative charge) and protons (positive charge) they are neutral overall. As zinc is a metal, it generally forms metallic compounds with. the electron configuration of zinc is: Zinc. Zinc Have A Negative Charge.