Define Crystal Lattice Class 12 . Crystal lattice and unit cell. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. If the radius of the cation is 110 pm,. The edge length of a face centered cubic cell of an ionic substance is 508 pm. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. Grasp the concept of crystal lattice with our. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. As a result, a lattice is a set of points arranged. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped.
from www.vedantu.com
The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. If the radius of the cation is 110 pm,. The edge length of a face centered cubic cell of an ionic substance is 508 pm. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. As a result, a lattice is a set of points arranged. Grasp the concept of crystal lattice with our. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. Crystal lattice and unit cell.
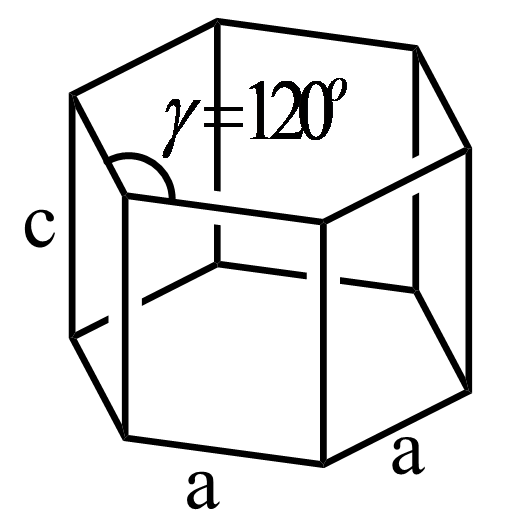
(a) Define crystal lattice.(b) What is the relation between the angles
Define Crystal Lattice Class 12 The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. As a result, a lattice is a set of points arranged. Grasp the concept of crystal lattice with our. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. If the radius of the cation is 110 pm,. The edge length of a face centered cubic cell of an ionic substance is 508 pm. Crystal lattice and unit cell. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped.
From www.scribd.com
crystal lattices PDF Define Crystal Lattice Class 12 As a result, a lattice is a set of points arranged. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. In the online videos lessons, our. Define Crystal Lattice Class 12.
From www.researchgate.net
c. 14 types of Brave crystal lattice Download Scientific Diagram Define Crystal Lattice Class 12 If the radius of the cation is 110 pm,. As a result, a lattice is a set of points arranged. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges. Define Crystal Lattice Class 12.
From www.studypool.com
SOLUTION Crystal lattice class 12 solid state Studypool Define Crystal Lattice Class 12 The edge length of a face centered cubic cell of an ionic substance is 508 pm. If the radius of the cation is 110 pm,. Grasp the concept of crystal lattice with our. As a result, a lattice is a set of points arranged. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an. Define Crystal Lattice Class 12.
From www.youtube.com
Crystal Lattices and Unit cells/ Class 12/Solid State (Lecture3) YouTube Define Crystal Lattice Class 12 As a result, a lattice is a set of points arranged. If the radius of the cation is 110 pm,. Crystal lattice and unit cell. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement. Define Crystal Lattice Class 12.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID1990542 Define Crystal Lattice Class 12 In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. As a result, a lattice is a set of points arranged. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. Crystal lattice and unit cell. A lattice or a. Define Crystal Lattice Class 12.
From gamesmartz.com
Crystal Lattice Definition & Image GameSmartz Define Crystal Lattice Class 12 As a result, a lattice is a set of points arranged. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. Grasp the concept of crystal lattice with our. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The. Define Crystal Lattice Class 12.
From www.youtube.com
CLASS XII/CHEMISTRY/SOLID STATEIII/CRYSTAL LATTICE AND UNIT CELLS Define Crystal Lattice Class 12 The edge length of a face centered cubic cell of an ionic substance is 508 pm. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. As a result, a lattice is a set of points arranged. In the online videos lessons, our expert explains how a. Define Crystal Lattice Class 12.
From www.youtube.com
CRYSTAL LATTICE, LATTICE POINT, UNIT CELL SOLID STATE PART 3 Define Crystal Lattice Class 12 The edge length of a face centered cubic cell of an ionic substance is 508 pm. Crystal lattice and unit cell. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The unit cell of a crystal can be completely specified by three vectors, a, b, c. Define Crystal Lattice Class 12.
From school.careers360.com
crystal lattices and unit cells Overview, Structure, Properties & Uses Define Crystal Lattice Class 12 A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The crystal lattice is the array of points at the corners of. Define Crystal Lattice Class 12.
From www.animalia-life.club
Crystal Systems And Bravais Lattices Define Crystal Lattice Class 12 In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. If the radius of the cation is 110 pm,. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. As a result, a lattice is a set of points arranged.. Define Crystal Lattice Class 12.
From www.youtube.com
Class 12 Chemistry Crystal Lattices and Atoms in a Unit Cell in Chapter Define Crystal Lattice Class 12 Grasp the concept of crystal lattice with our. As a result, a lattice is a set of points arranged. The edge length of a face centered cubic cell of an ionic substance is 508 pm. If the radius of the cation is 110 pm,. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice. Define Crystal Lattice Class 12.
From byjus.com
Explain crystal lattice. Define Crystal Lattice Class 12 Crystal lattice and unit cell. As a result, a lattice is a set of points arranged. Grasp the concept of crystal lattice with our. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The crystal lattice is the array of points at the corners of all. Define Crystal Lattice Class 12.
From www.youtube.com
SOLID STATE MCQs Crystal Lattice Class 12 Chemistry CBSE Define Crystal Lattice Class 12 The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. Crystal lattice and unit cell. As a result, a lattice is a set of points arranged. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A. Define Crystal Lattice Class 12.
From www.vedantu.com
(a) Define crystal lattice.(b) What is the relation between the angles Define Crystal Lattice Class 12 Grasp the concept of crystal lattice with our. Crystal lattice and unit cell. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. As a result, a lattice is a set of points arranged. In the online videos lessons, our expert explains how a crystal lattice is. Define Crystal Lattice Class 12.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Define Crystal Lattice Class 12 Grasp the concept of crystal lattice with our. Crystal lattice and unit cell. As a result, a lattice is a set of points arranged. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. The edge length of a face centered cubic cell of an. Define Crystal Lattice Class 12.
From studylib.net
The Crystal Lattice Define Crystal Lattice Class 12 A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. If the radius of the cation is 110 pm,. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. As a result,. Define Crystal Lattice Class 12.
From eduinput.com
Difference between crystal lattice and lattice point Define Crystal Lattice Class 12 As a result, a lattice is a set of points arranged. In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. Grasp the concept of crystal lattice with our. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The. Define Crystal Lattice Class 12.
From www.youtube.com
Seven Crystal Systems of Bravais Space Lattices. YouTube Define Crystal Lattice Class 12 A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. Crystal lattice and unit cell. The edge length of a face centered cubic cell of an ionic. Define Crystal Lattice Class 12.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Define Crystal Lattice Class 12 The edge length of a face centered cubic cell of an ionic substance is 508 pm. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A. Define Crystal Lattice Class 12.
From study.com
Crystal Lattice Definition & Structure Video & Lesson Transcript Define Crystal Lattice Class 12 A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. The edge length of a face centered cubic cell of an ionic substance is 508 pm. If the radius of the cation is 110 pm,. A solid material in which the constituent particles (molecules, atoms,. Define Crystal Lattice Class 12.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Define Crystal Lattice Class 12 Crystal lattice and unit cell. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. As a result, a lattice is a set of points arranged. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the. Define Crystal Lattice Class 12.
From www.pinterest.com
Types of crystal lattice Solid State Class 12 Chapter 1 Define Crystal Lattice Class 12 Grasp the concept of crystal lattice with our. The edge length of a face centered cubic cell of an ionic substance is 508 pm. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The crystal lattice is the array of points at the corners of all. Define Crystal Lattice Class 12.
From www.youtube.com
Crystal lattice ClassXII YouTube Define Crystal Lattice Class 12 Crystal lattice and unit cell. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. Grasp the concept of crystal lattice with our. If the radius of the cation is 110 pm,. In the online videos lessons, our expert explains how a crystal lattice is formed with. Define Crystal Lattice Class 12.
From www.youtube.com
CRYSTAL LATTICE AND UNIT CELLS SOLID STATE HINDI EXPLANATION Define Crystal Lattice Class 12 Crystal lattice and unit cell. As a result, a lattice is a set of points arranged. If the radius of the cation is 110 pm,. Grasp the concept of crystal lattice with our. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A lattice or a crystal lattice is. Define Crystal Lattice Class 12.
From chemistryskills.com
Crystal Lattice Definition Chemistry Unit Cell Definition Chemistry Define Crystal Lattice Class 12 The edge length of a face centered cubic cell of an ionic substance is 508 pm. Grasp the concept of crystal lattice with our. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. If the radius of the cation is 110 pm,. The crystal. Define Crystal Lattice Class 12.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Define Crystal Lattice Class 12 The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. As a result, a lattice is a set of points arranged. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. Crystal lattice and. Define Crystal Lattice Class 12.
From www.wise2wisdom.org
Atomic Structure and Crystal Lattice Explained and Simplified Define Crystal Lattice Class 12 In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. Grasp the concept of crystal lattice with our. The unit cell of a crystal can be completely specified by three. Define Crystal Lattice Class 12.
From www.youtube.com
crystal lattice and unit cell class 12 /solid state/ NEET IIT JEE Define Crystal Lattice Class 12 The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. In the online videos lessons, our expert explains how a crystal lattice is formed with. Define Crystal Lattice Class 12.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Define Crystal Lattice Class 12 Crystal lattice and unit cell. Grasp the concept of crystal lattice with our. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. In the online videos. Define Crystal Lattice Class 12.
From www.studypool.com
SOLUTION Crystal lattice class 12 solid state Studypool Define Crystal Lattice Class 12 Crystal lattice and unit cell. A lattice or a crystal lattice is the formation of an asymmetrical 3d structural arrangement of ions, atoms, and molecules to form a unit cell. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. A solid material in which the constituent. Define Crystal Lattice Class 12.
From www.youtube.com
Lattice, Basis, Crystal System Crystal Structure Solid State Define Crystal Lattice Class 12 The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. Grasp the concept of crystal lattice with our. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. If the radius of the cation is 110 pm,.. Define Crystal Lattice Class 12.
From eduinput.com
Crystal lattice Definition, Types, examples, lattice point Define Crystal Lattice Class 12 As a result, a lattice is a set of points arranged. The edge length of a face centered cubic cell of an ionic substance is 508 pm. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. In the online videos lessons, our expert explains how a crystal lattice is. Define Crystal Lattice Class 12.
From www.slideserve.com
PPT Chapter 6 Chemical Bonds PowerPoint Presentation, free download Define Crystal Lattice Class 12 A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. Crystal lattice and unit cell. The unit cell of a crystal can be completely specified by three vectors, a, b, c that form the edges of a parallelepiped. In the online videos lessons, our expert explains how. Define Crystal Lattice Class 12.
From www.youtube.com
Crystal Lattice and Unit Cellbccfccend centered and simple unit cell Define Crystal Lattice Class 12 In the online videos lessons, our expert explains how a crystal lattice is formed with lattice points. If the radius of the cation is 110 pm,. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. Grasp the concept of crystal lattice with our. A solid material in which the. Define Crystal Lattice Class 12.
From bernieralice.hyperphp.com
Types Of Crystal Lattice Structure bernieralice Define Crystal Lattice Class 12 The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure. A solid material in which the constituent particles (molecules, atoms, or ions) are arranged in an ordered pattern extending in all three spatial. The edge length of a face centered cubic cell of an ionic substance is 508 pm. The. Define Crystal Lattice Class 12.