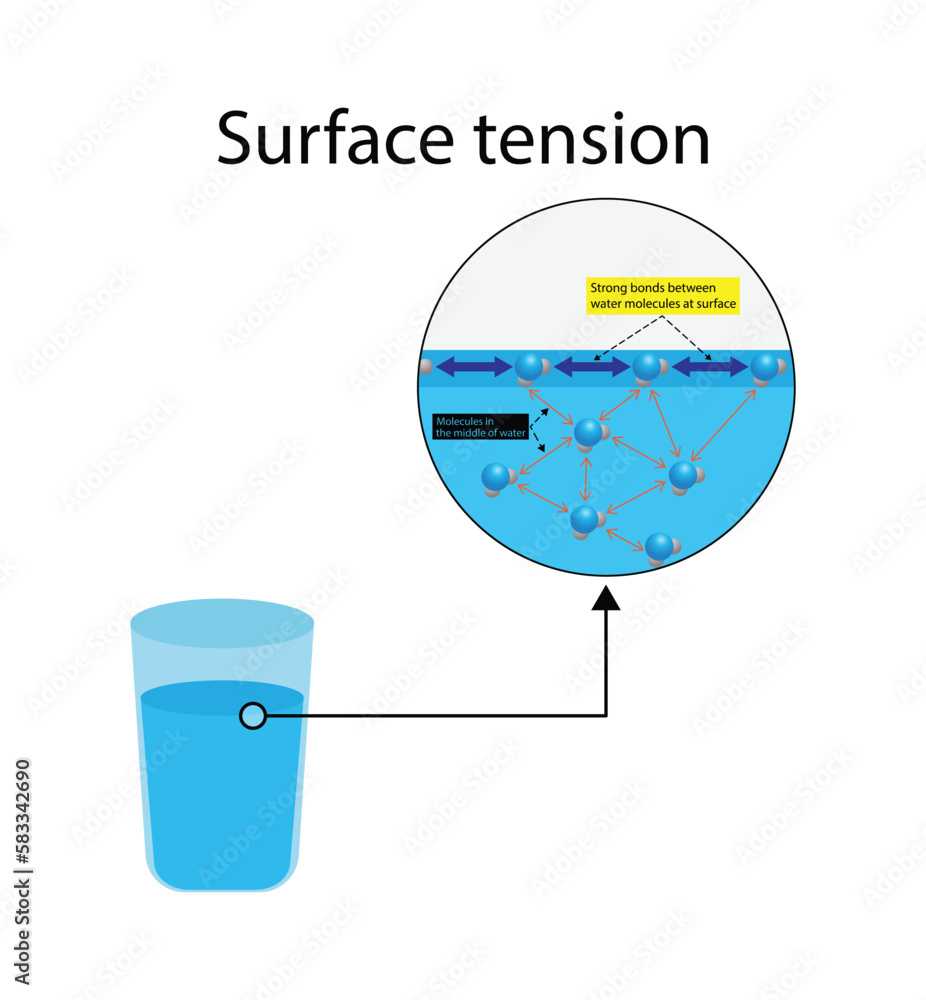
How To Determine Which Molecule Has Higher Surface Tension . The stronger the intermolecular interactions, the greater the surface tension. In this portion of the lab you will determine which liquid has the highest surface tension: Equivalently, it can be stated as surface energy in ergs per square centimeter. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Since these intermolecular forces vary. In order to do this, you will determine the number of. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water consists of one oxygen atom flanked by two hydrogen atoms. The surface tension of a liquid results from an imbalance of intermolecular. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The attraction of molecules at the surface of a liquid is called surface tension. Water, soapy water, or rubbing alcohol.
from stock.adobe.com
The attraction of molecules at the surface of a liquid is called surface tension. Since these intermolecular forces vary. In order to do this, you will determine the number of. Equivalently, it can be stated as surface energy in ergs per square centimeter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water, soapy water, or rubbing alcohol. In this portion of the lab you will determine which liquid has the highest surface tension: The surface tension of a liquid results from an imbalance of intermolecular. The stronger the intermolecular interactions, the greater the surface tension. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge.
illustration of physics, Surface tension of water, the cohesive forces
How To Determine Which Molecule Has Higher Surface Tension Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. In this portion of the lab you will determine which liquid has the highest surface tension: Water consists of one oxygen atom flanked by two hydrogen atoms. The attraction of molecules at the surface of a liquid is called surface tension. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs per square centimeter. In order to do this, you will determine the number of. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Water, soapy water, or rubbing alcohol. The surface tension of a liquid results from an imbalance of intermolecular.
From readchemistry.com
Determination of Surface Tension Read Chemistry How To Determine Which Molecule Has Higher Surface Tension Equivalently, it can be stated as surface energy in ergs per square centimeter. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of a liquid results from an imbalance of intermolecular. The stronger the intermolecular interactions, the. How To Determine Which Molecule Has Higher Surface Tension.
From smartquizbasket.blogspot.com
Smart Quiz Basket Surface Tension Definition Chemistry How To Determine Which Molecule Has Higher Surface Tension The attraction of molecules at the surface of a liquid is called surface tension. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In order to do this, you will determine the number of.. How To Determine Which Molecule Has Higher Surface Tension.
From universe-review.ca
Molecules How To Determine Which Molecule Has Higher Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In this portion of the lab you will determine which liquid has the highest surface tension: Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is the energy required to increase the surface area of a. How To Determine Which Molecule Has Higher Surface Tension.
From slidetodoc.com
BIOLOGICAL MOLECULES WATER HIGH SURFACE TENSION AND COHESION How To Determine Which Molecule Has Higher Surface Tension Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The attraction of molecules at the surface of a liquid is called surface tension. The stronger the intermolecular interactions, the greater the surface tension.. How To Determine Which Molecule Has Higher Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube How To Determine Which Molecule Has Higher Surface Tension The stronger the intermolecular interactions, the greater the surface tension. In order to do this, you will determine the number of. The polarity of water molecules can help explain why water has a strong surface tension. In this portion of the lab you will determine which liquid has the highest surface tension: The attraction of molecules at the surface of. How To Determine Which Molecule Has Higher Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How To Determine Which Molecule Has Higher Surface Tension The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water molecules have a dipole, meaning that one side of. How To Determine Which Molecule Has Higher Surface Tension.
From www.pinterest.com
Surface tension, Surface, Tension How To Determine Which Molecule Has Higher Surface Tension The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface tension. Water, soapy water, or rubbing alcohol. Surface tension is measured as. How To Determine Which Molecule Has Higher Surface Tension.
From mavink.com
Water Molecules Surface Tension How To Determine Which Molecule Has Higher Surface Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water, soapy water, or rubbing. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint How To Determine Which Molecule Has Higher Surface Tension In order to do this, you will determine the number of. The polarity of water molecules can help explain why water has a strong surface tension. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Water consists of one oxygen atom flanked by two. How To Determine Which Molecule Has Higher Surface Tension.
From mungfali.com
Surface Tension Chart How To Determine Which Molecule Has Higher Surface Tension Water consists of one oxygen atom flanked by two hydrogen atoms. Equivalently, it can be stated as surface energy in ergs per square centimeter. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary. The polarity of water molecules can help explain why water has a strong surface tension.. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How To Determine Which Molecule Has Higher Surface Tension The polarity of water molecules can help explain why water has a strong surface tension. Equivalently, it can be stated as surface energy in ergs per square centimeter. The stronger the intermolecular interactions, the greater the surface tension. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water molecules have a dipole, meaning that. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Forces of attraction/repulsion between molecules. PowerPoint How To Determine Which Molecule Has Higher Surface Tension The surface tension of a liquid results from an imbalance of intermolecular. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Surface tension. How To Determine Which Molecule Has Higher Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? How To Determine Which Molecule Has Higher Surface Tension Water, soapy water, or rubbing alcohol. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In this portion of the lab you will determine which liquid has the. How To Determine Which Molecule Has Higher Surface Tension.
From www.numerade.com
SOLVED Which of the following molecules would have the highest surface How To Determine Which Molecule Has Higher Surface Tension The surface tension of a liquid results from an imbalance of intermolecular. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In order to do this, you will determine the number of. Water consists. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 How To Determine Which Molecule Has Higher Surface Tension The stronger the intermolecular interactions, the greater the surface tension. Equivalently, it can be stated as surface energy in ergs per square centimeter. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface tension is the energy required to increase the surface area. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID How To Determine Which Molecule Has Higher Surface Tension Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. In this portion of the lab you will determine which liquid has the highest surface tension: Since these intermolecular forces vary. Surface tension is typically measured in dynes/cm, the force in dynes required to break. How To Determine Which Molecule Has Higher Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. How To Determine Which Molecule Has Higher Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Since these intermolecular forces vary. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. In this portion of the lab you will determine which liquid has the highest. How To Determine Which Molecule Has Higher Surface Tension.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube How To Determine Which Molecule Has Higher Surface Tension Water, soapy water, or rubbing alcohol. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. The surface tension of a liquid results from an imbalance of intermolecular. In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this,. How To Determine Which Molecule Has Higher Surface Tension.
From learningschooltrkesp5v.z22.web.core.windows.net
Viscosity Vs Surface Tension How To Determine Which Molecule Has Higher Surface Tension In order to do this, you will determine the number of. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid is called surface tension. Since these intermolecular forces vary.. How To Determine Which Molecule Has Higher Surface Tension.
From recemev.jimdo.com
Surface Tension recemev How To Determine Which Molecule Has Higher Surface Tension Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Since these intermolecular forces vary. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Surface tension is typically measured in dynes/cm, the force in dynes required to break a. How To Determine Which Molecule Has Higher Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How To Determine Which Molecule Has Higher Surface Tension Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. The surface tension of a liquid results from an imbalance of intermolecular. In order to do this, you will determine the number of. Water consists of one oxygen atom flanked by two hydrogen atoms. Surface. How To Determine Which Molecule Has Higher Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How To Determine Which Molecule Has Higher Surface Tension The surface tension of a liquid results from an imbalance of intermolecular. The polarity of water molecules can help explain why water has a strong surface tension. Since these intermolecular forces vary. Water consists of one oxygen atom flanked by two hydrogen atoms. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding Covalent molecules PowerPoint How To Determine Which Molecule Has Higher Surface Tension In this portion of the lab you will determine which liquid has the highest surface tension: The stronger the intermolecular interactions, the greater the surface tension. The polarity of water molecules can help explain why water has a strong surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Water. How To Determine Which Molecule Has Higher Surface Tension.
From courses.lumenlearning.com
Properties of Liquids Chemistry Atoms First How To Determine Which Molecule Has Higher Surface Tension The surface tension of a liquid results from an imbalance of intermolecular. Water consists of one oxygen atom flanked by two hydrogen atoms. The polarity of water molecules can help explain why water has a strong surface tension. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is typically measured in dynes/cm, the. How To Determine Which Molecule Has Higher Surface Tension.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American How To Determine Which Molecule Has Higher Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is measured as the energy required to increase the surface area of a liquid by a unit of area. Water, soapy water, or rubbing alcohol. Surface tension is the energy, or work, required to increase the surface area of a. How To Determine Which Molecule Has Higher Surface Tension.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co How To Determine Which Molecule Has Higher Surface Tension Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge. Since these intermolecular forces vary. Water, soapy water, or rubbing alcohol. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. The surface tension of a liquid results from an. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How To Determine Which Molecule Has Higher Surface Tension Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension of a liquid results from an imbalance of intermolecular. Since these intermolecular forces vary. Water molecules have a dipole, meaning that one side of the molecule has a slightly positive charge and one part has a slightly negative charge.. How To Determine Which Molecule Has Higher Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How To Determine Which Molecule Has Higher Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the greater the surface tension. Equivalently, it can be stated as surface energy in ergs. How To Determine Which Molecule Has Higher Surface Tension.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts How To Determine Which Molecule Has Higher Surface Tension The attraction of molecules at the surface of a liquid is called surface tension. Equivalently, it can be stated as surface energy in ergs per square centimeter. The surface tension of a liquid results from an imbalance of intermolecular. In this portion of the lab you will determine which liquid has the highest surface tension: In order to do this,. How To Determine Which Molecule Has Higher Surface Tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How To Determine Which Molecule Has Higher Surface Tension In this portion of the lab you will determine which liquid has the highest surface tension: The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid is called surface tension. The surface tension of a liquid results from an imbalance of intermolecular. In order to do this, you will determine. How To Determine Which Molecule Has Higher Surface Tension.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download How To Determine Which Molecule Has Higher Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. In this portion of the lab you will determine which liquid has the highest surface tension: The stronger the intermolecular interactions, the greater the surface tension. The surface tension of a liquid results from an imbalance of intermolecular. Surface tension. How To Determine Which Molecule Has Higher Surface Tension.
From brainly.in
1. Define surface tension. Write formula, unit and dimension of surface How To Determine Which Molecule Has Higher Surface Tension The polarity of water molecules can help explain why water has a strong surface tension. The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. The. How To Determine Which Molecule Has Higher Surface Tension.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How To Determine Which Molecule Has Higher Surface Tension Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. The surface tension of a liquid results from an imbalance of intermolecular. The attraction of molecules at the surface of a liquid is called surface tension. In this portion of the lab you will determine which liquid has the highest. How To Determine Which Molecule Has Higher Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How To Determine Which Molecule Has Higher Surface Tension In order to do this, you will determine the number of. Surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Water consists of one oxygen atom flanked by two hydrogen atoms. Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is. How To Determine Which Molecule Has Higher Surface Tension.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How To Determine Which Molecule Has Higher Surface Tension Water, soapy water, or rubbing alcohol. Water consists of one oxygen atom flanked by two hydrogen atoms. The stronger the intermolecular interactions, the greater the surface tension. Equivalently, it can be stated as surface energy in ergs per square centimeter. The polarity of water molecules can help explain why water has a strong surface tension. Water molecules have a dipole,. How To Determine Which Molecule Has Higher Surface Tension.