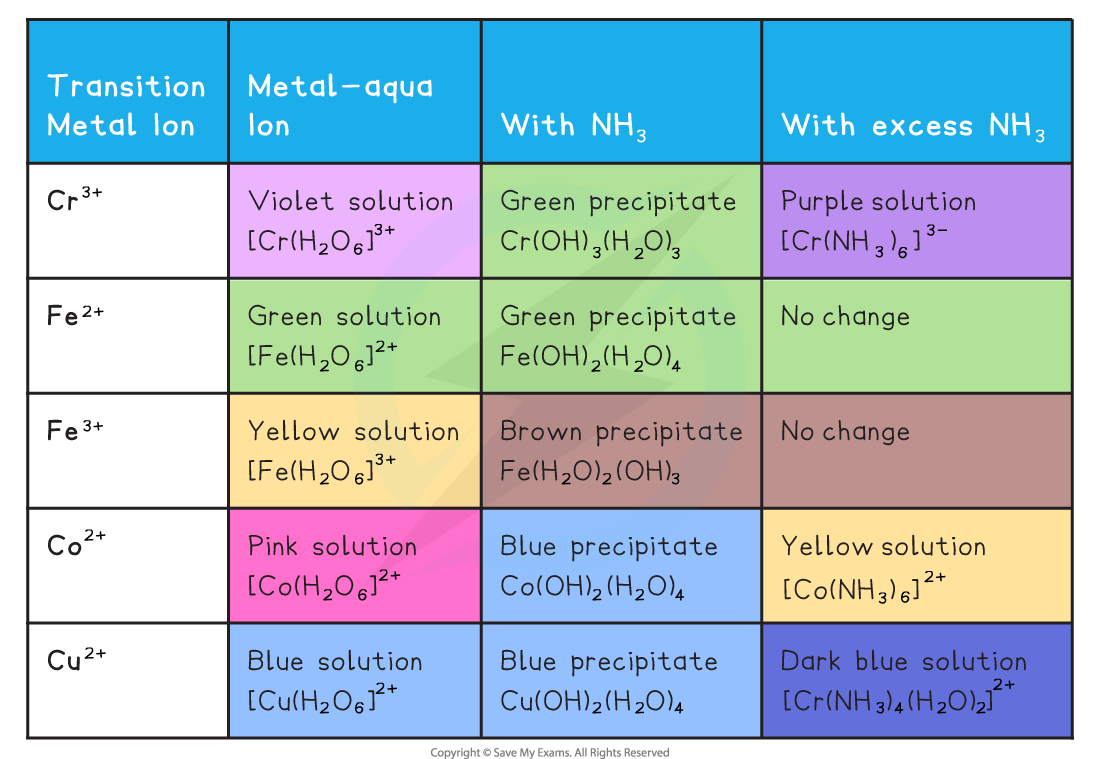
Zinc Complex Ion With Ammonia . As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Zinc(ii) ion forms complex ions readily. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Because it is a stronger. Complexation of zinc ion with ammonia. Because it is a stronger base. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc +2 ion and dilute ammonia solution reaction. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution.
from www.hanlin.com
Complexation of zinc ion with ammonia. Because it is a stronger. Because it is a stronger base. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Solution翰林国际教育
Zinc Complex Ion With Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Zinc(ii) ion forms complex ions readily. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). Because it is a stronger base. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Complexation of zinc ion with ammonia. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Because it is a stronger. Zinc +2 ion and dilute ammonia solution reaction. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq).
From pixels.com
Zinc Sulphate In Ammonia Solution Photograph by Martyn F. Chillmaid/science Photo Library Pixels Zinc Complex Ion With Ammonia Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Because it is a stronger. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia. Zinc Complex Ion With Ammonia.
From www.coursehero.com
[Solved] 1. Ammonia to produce nitrogen and hydrogen 2. Zinc and... Course Hero Zinc Complex Ion With Ammonia When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution,. Zinc Complex Ion With Ammonia.
From www.guidechem.com
complex 67859512 wiki Zinc Complex Ion With Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Complexation of zinc ion with ammonia. Zinc(ii) ion forms complex ions readily. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Because it is a stronger base. As an example of the formation of complex ions, consider the addition of ammonia to an. Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVEDThe solubility of zinc oxalate, ZnC2 O4, in 0.0150 M ammonia is 3.6 ×10^4 mol / L. What Zinc Complex Ion With Ammonia Complexation of zinc ion with ammonia. Zinc(ii) ion forms complex ions readily. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As an example of the formation. Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVED Consider the insoluble compound zinc sulfide, ZnS. The zinc ion also forms a complex Zinc Complex Ion With Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh). Zinc Complex Ion With Ammonia.
From www.chemistrystudent.com
Ligand Substitution (ALevel) ChemistryStudents Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Because it is a stronger. Because it is a stronger base. As an example of the formation of complex ions, consider the addition. Zinc Complex Ion With Ammonia.
From www.numerade.com
In aqueous solution, the Cd2+ ion forms a complex with four ammonia molecules. Zinc Complex Ion With Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Complexation of zinc ion with ammonia. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate). Zinc Complex Ion With Ammonia.
From www.researchgate.net
Hypothetic molecular structure of resulting Zinc complex of... Download Scientific Diagram Zinc Complex Ion With Ammonia \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Because it is a stronger. Because it is a stronger base. Zinc +2 ion and dilute ammonia solution reaction. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the. Zinc Complex Ion With Ammonia.
From almerja.net
Ligand Exchange of hexaaquacobalt(II) ions with ammonia solution Zinc Complex Ion With Ammonia When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Zinc(ii) ion. Zinc Complex Ion With Ammonia.
From www.researchgate.net
Synthesis of the ligand, H2ATSE, and the zinc complex, [Zn‐ATSE]2 Download Scientific Diagram Zinc Complex Ion With Ammonia Complexation of zinc ion with ammonia. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Because it is a stronger base. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate). Zinc Complex Ion With Ammonia.
From onlinelibrary.wiley.com
Visible Light Responsive Dinuclear Zinc Complex Consisting of Proximally Arranged Two d10‐Zinc Zinc Complex Ion With Ammonia Because it is a stronger. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Because it is a stronger base. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. Zinc(ii) ion reacts. Zinc Complex Ion With Ammonia.
From www.sciencephoto.com
Zinc sulphate in ammonia solution Stock Image C016/9564 Science Photo Library Zinc Complex Ion With Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. \[\ce{zn^{2+}(aq) +. Zinc Complex Ion With Ammonia.
From www.researchgate.net
Speciation diagram of the zinc complex ions. Download Scientific Diagram Zinc Complex Ion With Ammonia Because it is a stronger base. Zinc +2 ion and dilute ammonia solution reaction. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4.. Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVEDIn a complex ion, a central ruthenium ion, Ru(III), is bonded to six ammonia molecules Zinc Complex Ion With Ammonia Because it is a stronger base. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Zinc +2 ion and dilute ammonia solution reaction. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). Complexation of zinc ion with ammonia. Zinc(ii) ion forms complex ions readily. This page describes and explains the reactions between. Zinc Complex Ion With Ammonia.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Solution翰林国际教育 Zinc Complex Ion With Ammonia Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Zinc +2 ion and dilute ammonia solution reaction. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Zinc(ii). Zinc Complex Ion With Ammonia.
From igennus.com
High Absorption Zinc Complex 25mg with Copper, Chelated Zinc Picolinat Igennus Healthcare Zinc Complex Ion With Ammonia \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc +2 ion and dilute ammonia solution reaction. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Because it is a stronger.. Zinc Complex Ion With Ammonia.
From www.researchgate.net
Structure and mass spectral study of zinc complex. Chemical structure... Download Scientific Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h. Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The zinc ion also forms a Zinc Complex Ion With Ammonia This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). Complexation of zinc ion with ammonia. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. When aqueous dilute ammonia solution is added. Zinc Complex Ion With Ammonia.
From www.researchgate.net
Scheme 3 Synthesis of magnesium and zinc complexes 1b9b. Download Scientific Diagram Zinc Complex Ion With Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Complexation of zinc ion with ammonia. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h. Zinc Complex Ion With Ammonia.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Complexation of zinc ion with ammonia. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Zinc +2 ion and dilute ammonia solution reaction. When aqueous dilute ammonia solution is added slowly. Zinc Complex Ion With Ammonia.
From www.chemistryviews.org
Zinc Anilide Complex for the SemiHydrogenation of Alkynes ChemistryViews Zinc Complex Ion With Ammonia Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Because it is a stronger base. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). Zinc(ii) ion forms complex ions readily. When aqueous dilute ammonia solution is added slowly to the colourless zn. Zinc Complex Ion With Ammonia.
From 2012books.lardbucket.org
The Formation of Complex Ions Zinc Complex Ion With Ammonia When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to. Zinc Complex Ion With Ammonia.
From www.doubtnut.com
A complex of platinum, ammonia and chloride produces four ions per mol Zinc Complex Ion With Ammonia When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Zinc(ii) ion forms complex ions readily. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Complexation of zinc ion with ammonia. This. Zinc Complex Ion With Ammonia.
From www.chegg.com
Solved Table 3. Formation Constants of Complex Ions Cation Zinc Complex Ion With Ammonia Complexation of zinc ion with ammonia. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc +2 ion and dilute ammonia solution reaction. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. Zinc(ii) ion forms complex ions readily. When aqueous. Zinc Complex Ion With Ammonia.
From www.colorado.edu
E740 Equilibrium Complex Ions Metal + Ammonia Complexes Lecture Demonstration Manual Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. \[\ce{zn^{2+}(aq) +. Zinc Complex Ion With Ammonia.
From www.mdpi.com
Crystals Free FullText Leaching of Secondary Zinc Oxide in a NH3NH4HCO3H2O System Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh). \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Because it is a stronger. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Complexation. Zinc Complex Ion With Ammonia.
From www.chemistrystudent.com
Complex Ions (ALevel) ChemistryStudent Zinc Complex Ion With Ammonia When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. This page describes and explains the reactions between. Zinc Complex Ion With Ammonia.
From www.mdpi.com
Crystals Free FullText Synthesis of a Novel Zinc(II) Porphyrin Complex, Halide Ion Zinc Complex Ion With Ammonia Complexation of zinc ion with ammonia. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2. Zinc Complex Ion With Ammonia.
From www.youtube.com
Test for Zinc ion with Aqueous Ammonia YouTube Zinc Complex Ion With Ammonia This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. Because it is a stronger. Complexation of zinc ion with ammonia. When zinc ion reacts with ammonia, the first product is zinc hydroxide. Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide En(OH) The zinc ionalso forns complex Zinc Complex Ion With Ammonia Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. Zinc +2 ion and dilute ammonia solution reaction. Because it is a stronger. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Zinc Complex Ion With Ammonia.
From www.numerade.com
SOLVEDThe reaction of a drycell battery may be represented as follows zinc reacts with Zinc Complex Ion With Ammonia Complexation of zinc ion with ammonia. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc(ii) ion forms. Zinc Complex Ion With Ammonia.
From www.dlt.ncssm.edu
TIGER NCSSM Distance Education and Extended Programs Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. Because it is a stronger. Complexation of zinc ion with ammonia. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu. Zinc Complex Ion With Ammonia.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Reaction between the Ammonium Zinc Complex Ion With Ammonia Zinc(ii) ion forms complex ions readily. Because it is a stronger. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. When aqueous dilute ammonia solution is added slowly to the colourless zn 2+ solution, a white. This page describes and explains the reactions between complex ions of the type [m(h 2 o) 6] n+ and ammonia solution. As an example of the formation. Zinc Complex Ion With Ammonia.
From www.yumpu.com
ZincEDTA Complexation Reaction Zinc Complex Ion With Ammonia Because it is a stronger base. Reaction of zinc with ammonia zn(ii) is precipitated by ammonia ions as zn(oh) 2 (white amorphous precipitate) zn 2+ (aq) + 2 nh 3 (aq). Zinc +2 ion and dilute ammonia solution reaction. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: As an example of the formation of complex ions, consider. Zinc Complex Ion With Ammonia.
From www.youtube.com
What happens when ammonia solution is added first dropwise and then in excess to zinc sulphate Zinc Complex Ion With Ammonia As an example of the formation of complex ions, consider the addition of ammonia to an aqueous solution of the hydrated cu 2+ ion { [cu (h 2 o) 6] 2+}. When zinc ion reacts with ammonia, the first product is zinc hydroxide (compare with dic4. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc(ii) ion reacts. Zinc Complex Ion With Ammonia.