Explain Electrochemical Corrosion . corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. this section provides an overview of several of the more common electrochemical corrosion measurement.
from www.doubtnut.com
this section provides an overview of several of the more common electrochemical corrosion measurement. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. Large sums of money are spent each year repairing the. corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the.
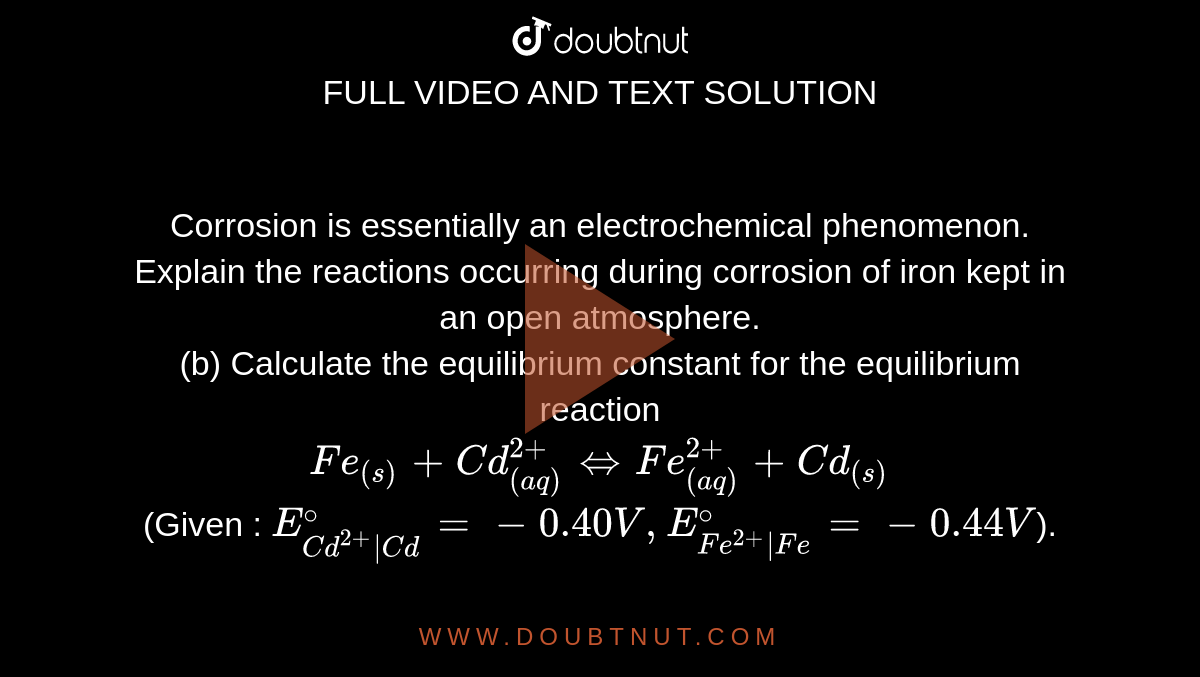
Corrosion is essentially an electrochemical phenomenon. Explain the
Explain Electrochemical Corrosion electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion measurement. corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. Large sums of money are spent each year repairing the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the.
From library.fiveable.me
Corrosion Intro to Chemistry Study Guide 2024 Fiveable Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. this section provides an overview of several of the more common electrochemical corrosion measurement. . Explain Electrochemical Corrosion.
From knowledge.electrochem.org
Electrochemistry Encyclopedia Electrochemistry of corrosion Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. this section provides an overview of several of the more common electrochemical corrosion measurement. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in. Explain Electrochemical Corrosion.
From www.mdpi.com
Applied Sciences Free FullText Recent Trends and Progress in Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. this section provides an overview of several of the more common electrochemical corrosion measurement. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the large variety of electrolytes found. Explain Electrochemical Corrosion.
From www.youtube.com
Electrochemical theory of corrosion YouTube Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the. Explain Electrochemical Corrosion.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the. Explain Electrochemical Corrosion.
From www.meritnation.com
The chemistry of corrosion of iron is essentially an electrochemical Explain Electrochemical Corrosion Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. this section provides an overview of several of the more common electrochemical corrosion measurement. if an electrochemical reaction occurs on a metal surface, then this leads to. Explain Electrochemical Corrosion.
From www.studypool.com
SOLUTION Type of electrochemical corrosion Studypool Explain Electrochemical Corrosion electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion measurement. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. Large sums of money are spent each year. Explain Electrochemical Corrosion.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. . Explain Electrochemical Corrosion.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the large variety of. Explain Electrochemical Corrosion.
From www.researchgate.net
(PDF) Fundamentals of Electrochemistry, Corrosion and Corrosion Protection Explain Electrochemical Corrosion Large sums of money are spent each year repairing the. corrosion is the degradation of a metal caused by an electrochemical process. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. this section provides an overview of several of the more common electrochemical corrosion measurement. electrochemical. Explain Electrochemical Corrosion.
From www.youtube.com
Hydrogen evolution and Oxygen Absorption Mechanism of wet corrosion Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. Large sums of money are spent each year repairing the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion. Explain Electrochemical Corrosion.
From www.researchgate.net
Electrochemical impedance spectra of mild steel corrosion in 0.5 M H 2 Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs. Explain Electrochemical Corrosion.
From www.youtube.com
Part 1 Corrosion in refineries, Electrochemical & dry corrosion Explain Electrochemical Corrosion Large sums of money are spent each year repairing the. corrosion is the degradation of a metal caused by an electrochemical process. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. . Explain Electrochemical Corrosion.
From saylordotorg.github.io
Describing Electrochemical Cells Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. Large sums of money are spent each year repairing the.. Explain Electrochemical Corrosion.
From www.studocu.com
DOC20230222WA0008 Reference Chapter Electrochemistry and Explain Electrochemical Corrosion Large sums of money are spent each year repairing the. corrosion is the degradation of a metal caused by an electrochemical process. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. this section provides an overview of several of the more common electrochemical corrosion measurement. the. Explain Electrochemical Corrosion.
From www.researchgate.net
Electrochemical corrosion behavior of the Zn alloys. A) OCP variation Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. corrosion is the degradation of a metal caused by an electrochemical process. if an electrochemical reaction occurs on a metal surface, then. Explain Electrochemical Corrosion.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Explain Electrochemical Corrosion the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. corrosion is. Explain Electrochemical Corrosion.
From www.myxxgirl.com
Electrochemistry Class Chemistry Commercial Cells And Corrosion My Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. Large sums of money are spent each year repairing the. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. . Explain Electrochemical Corrosion.
From www.comsol.it
Modeling Pitting Corrosion in COMSOL Multiphysics® COMSOL Blog Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. this section provides an overview of several of the more common electrochemical corrosion measurement. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. if an electrochemical reaction occurs. Explain Electrochemical Corrosion.
From chem.libretexts.org
Chapter 19.6 Corrosion Chemistry LibreTexts Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. corrosion is the degradation of a metal caused by an electrochemical process. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in. Explain Electrochemical Corrosion.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. Large sums of. Explain Electrochemical Corrosion.
From www.doubtnut.com
Corrosion is essentially an electrochemical phenomenon. Explain the Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. corrosion is the degradation of a metal caused by an electrochemical process. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. this section provides an overview. Explain Electrochemical Corrosion.
From www.researchgate.net
Scheme illustrating (a) the electrochemical corrosion process at a Explain Electrochemical Corrosion the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion measurement. if an electrochemical reaction occurs on a. Explain Electrochemical Corrosion.
From askfilo.com
cell. What is Corrosion ? Explain electrochemical theory of rusting. 3. H.. Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. this section provides an overview of several of the more common electrochemical corrosion measurement. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes. Explain Electrochemical Corrosion.
From www.youtube.com
Electrochemical Corrosion YouTube Explain Electrochemical Corrosion Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. corrosion is the degradation of a metal caused by an electrochemical process. this section provides an overview of several of the more common electrochemical corrosion measurement. . Explain Electrochemical Corrosion.
From www.mdpi.com
Applied Sciences Free FullText Recent Trends and Progress in Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. corrosion is the degradation of a metal caused by an electrochemical process. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. the corrosion rate is enhanced by an electrochemical process in. Explain Electrochemical Corrosion.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. . Explain Electrochemical Corrosion.
From www.slideserve.com
PPT Corrosion of Metals PowerPoint Presentation, free download ID Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. Large sums of money are spent each year repairing the. if an electrochemical reaction occurs on a metal surface, then this leads to. Explain Electrochemical Corrosion.
From www.mdpi.com
Applied Sciences Free FullText Recent Trends and Progress in Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. Large sums of money are spent each year repairing the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell. Explain Electrochemical Corrosion.
From dokumen.tips
(PDF) Define corrosion. Explain the electrochemical theory of corrosion Explain Electrochemical Corrosion electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion measurement. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. Large sums of money are spent each year repairing. Explain Electrochemical Corrosion.
From www.researchgate.net
Schematic diagram of electrochemical corrosion mechanism Download Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. corrosion is the degradation of a metal caused by an electrochemical process. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. the corrosion rate is enhanced by an electrochemical process in which. Explain Electrochemical Corrosion.
From www.researchgate.net
The electrochemical corrosion surface morphology images of a) and b Explain Electrochemical Corrosion corrosion is the degradation of a metal caused by an electrochemical process. Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration. Explain Electrochemical Corrosion.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu Explain Electrochemical Corrosion if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. this section provides an overview of several of the more common electrochemical corrosion measurement. corrosion is the degradation of a metal caused. Explain Electrochemical Corrosion.
From www.studypool.com
SOLUTION Electrochemistry and corrosion Studypool Explain Electrochemical Corrosion the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to metal deterioration or degradation and the. electrochemical corrosion occurs in the large variety of electrolytes found in natural environments and industrial. Large sums of. Explain Electrochemical Corrosion.
From www.semanticscholar.org
Figure 1 from Electrochemical Corrosion Properties of Amorphous CoNbB Explain Electrochemical Corrosion this section provides an overview of several of the more common electrochemical corrosion measurement. Large sums of money are spent each year repairing the. the corrosion rate is enhanced by an electrochemical process in which a water droplet becomes a voltaic cell in contact. if an electrochemical reaction occurs on a metal surface, then this leads to. Explain Electrochemical Corrosion.