Safety Analysis Clinical Trials . frequent and thorough monitoring of patient safety is a requirement of clinical trials research. monitoring patient safety during clinical trials is a critical component throughout the drug development life. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data.
from www.cff.org
randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated.
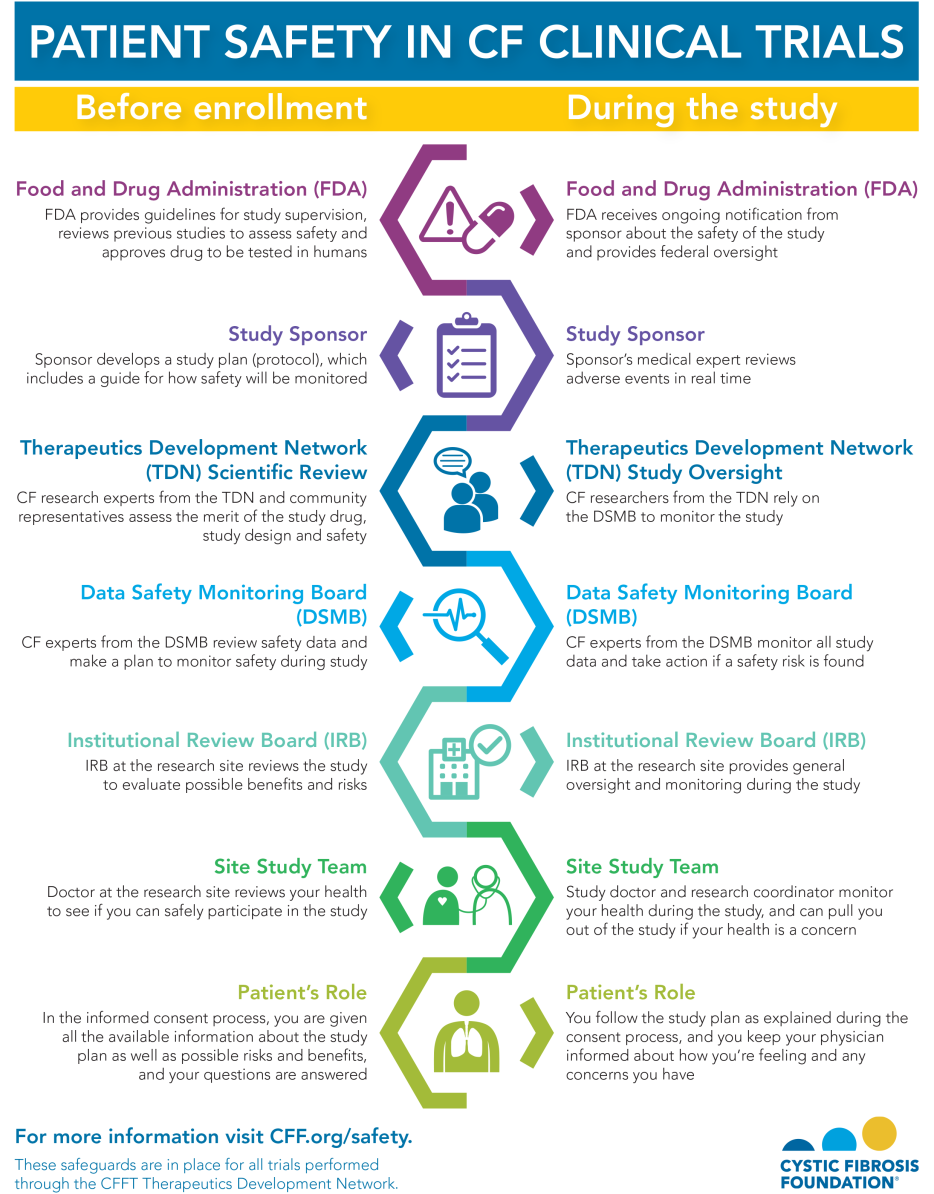
Patient Safety Is a Top Priority in Clinical Research Cystic Fibrosis
Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research.
From www.knoell.com
Clinical Safety knoell Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a. Safety Analysis Clinical Trials.
From issuu.com
Risk Assessment in Clinical Trial by adamsnelson Issuu Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting,. Safety Analysis Clinical Trials.
From pharmabeej.com
Patient Safety In Clinical Trials Pharmabeej Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From www.tldrpharmacy.com
A Guide to Clinical Trial Endpoints — tl;dr pharmacy Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From www.youtube.com
Patient Safety in Clinical Trials YouTube Safety Analysis Clinical Trials monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is. Safety Analysis Clinical Trials.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From www.slideserve.com
PPT SAFETY MONITORING IN CLINICAL TRIALS PowerPoint Presentation Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the. Safety Analysis Clinical Trials.
From www.asphalion.com
Elaboration of safety management plan for clinical trials key points Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From www.yumpu.com
safety reporting flowchart (PDF, 86.41 KB) Clinical Trials Toolkit Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. monitoring patient safety during clinical trials is a critical component throughout. Safety Analysis Clinical Trials.
From pharmrev.aspetjournals.org
Development of Novel, ValueBased, Digital Endpoints for Clinical Safety Analysis Clinical Trials monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the. Safety Analysis Clinical Trials.
From mapaseagame.blogspot.com
Efficacy Analysis Clinical Trials mapasgmaes Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. monitoring patient safety during clinical trials is a critical component throughout. Safety Analysis Clinical Trials.
From www.aging-us.com
The efficacy and safety of PD1/PDL1 immune checkpoint inhibitors in Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the. Safety Analysis Clinical Trials.
From www.hemophilia.org
Understanding Clinical Trials NBDF Safety Analysis Clinical Trials monitoring patient safety during clinical trials is a critical component throughout the drug development life. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. the program safety analysis plan (psap) was proposed previously as a tool. Safety Analysis Clinical Trials.
From www.aging-us.com
The efficacy and safety of PD1/PDL1 immune checkpoint inhibitors in Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout. Safety Analysis Clinical Trials.
From www.quantics.co.uk
What is a Safety Population in a Clinical Trial? Quantics Biostatistics Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. randomized clinical trials are the gold standard for evaluating. Safety Analysis Clinical Trials.
From www.cff.org
Patient Safety Is a Top Priority in Clinical Research Cystic Fibrosis Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. monitoring patient safety during clinical trials is a critical. Safety Analysis Clinical Trials.
From researchsapling.blogspot.com
Clinical Research WHAT CAN WE DERIVE BY HAZARD RATIO INTERPRETATION IN Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating. Safety Analysis Clinical Trials.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a. Safety Analysis Clinical Trials.
From www.xfanzexpo.com
Pdf) Safety Monitoring In Clinical Trials throughout Dsmb Report Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the reporting, analysis. Safety Analysis Clinical Trials.
From dxondhmuy.blob.core.windows.net
Drug Safety Assessment In Clinical Trials at Maude Smith blog Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. frequent and thorough monitoring of patient safety is a. Safety Analysis Clinical Trials.
From www.jmp.com
RiskBased Monitoring in Clinical Trials Getting Started JMP Clinical Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting,. Safety Analysis Clinical Trials.
From www.slideserve.com
PPT Safety Part I Clinical Trial Safety and Safety Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. monitoring patient safety during clinical trials is a critical component throughout. Safety Analysis Clinical Trials.
From www.uhs.nhs.uk
What is clinical research? Safety Analysis Clinical Trials monitoring patient safety during clinical trials is a critical component throughout the drug development life. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. we summarize the principal methodological challenges in the reporting, analysis and. Safety Analysis Clinical Trials.
From elearning.wfh.org
Monitoring Patient Safety in Clinical Trials eLearning Platform Safety Analysis Clinical Trials monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a tool. Safety Analysis Clinical Trials.
From lustgarten.org
Clinical Trial Phases Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From clinosolresearch.blogspot.com
DATA SAFETY MONITORING BOARD IN CLINICAL TRIALS Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. frequent and thorough monitoring of patient safety is. Safety Analysis Clinical Trials.
From www.slideserve.com
PPT Safety Reporting IN Clinical Trials PowerPoint Presentation, free Safety Analysis Clinical Trials the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a. Safety Analysis Clinical Trials.
From www.researchgate.net
An Approach to Safety Analysis of Clinical Workflows [3] Download Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. the program safety analysis plan (psap) was proposed previously as a tool. Safety Analysis Clinical Trials.
From prorelixresearch.com
Planning Clinical Trial in USA? Here is what you should know Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. frequent and thorough monitoring of patient safety is. Safety Analysis Clinical Trials.
From www.cloudbyz.com
Benefits of RiskBased Monitoring in Clinical Trials Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. frequent and thorough monitoring of patient safety is. Safety Analysis Clinical Trials.
From dxondhmuy.blob.core.windows.net
Drug Safety Assessment In Clinical Trials at Maude Smith blog Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a. Safety Analysis Clinical Trials.
From www.slideserve.com
PPT SAFETY MONITORING IN CLINICAL TRIALS PowerPoint Presentation Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. randomized clinical trials are the gold standard for evaluating the benefits and. Safety Analysis Clinical Trials.
From research.unsw.edu.au
Safety Monitoring and Reporting in UNSW Sponsored Clinical Trials Safety Analysis Clinical Trials frequent and thorough monitoring of patient safety is a requirement of clinical trials research. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. monitoring patient safety during clinical trials is a critical component throughout the drug development life. we summarize the principal methodological challenges in the reporting, analysis. Safety Analysis Clinical Trials.
From pharmdguru.com
16. SAFETY MONITORING IN CLINICAL TRIALS PHARMD GURU Safety Analysis Clinical Trials we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. the program safety analysis plan (psap) was proposed previously as a tool to proactively plan for integrated. frequent and thorough monitoring of patient safety is a. Safety Analysis Clinical Trials.
From www.lymphoma.org.au
Understanding Clinical Trials Lymphoma Australia Safety Analysis Clinical Trials randomized clinical trials are the gold standard for evaluating the benefits and harms of medical interventions. we summarize the principal methodological challenges in the reporting, analysis and interpretation of safety data. monitoring patient safety during clinical trials is a critical component throughout the drug development life. frequent and thorough monitoring of patient safety is a requirement. Safety Analysis Clinical Trials.