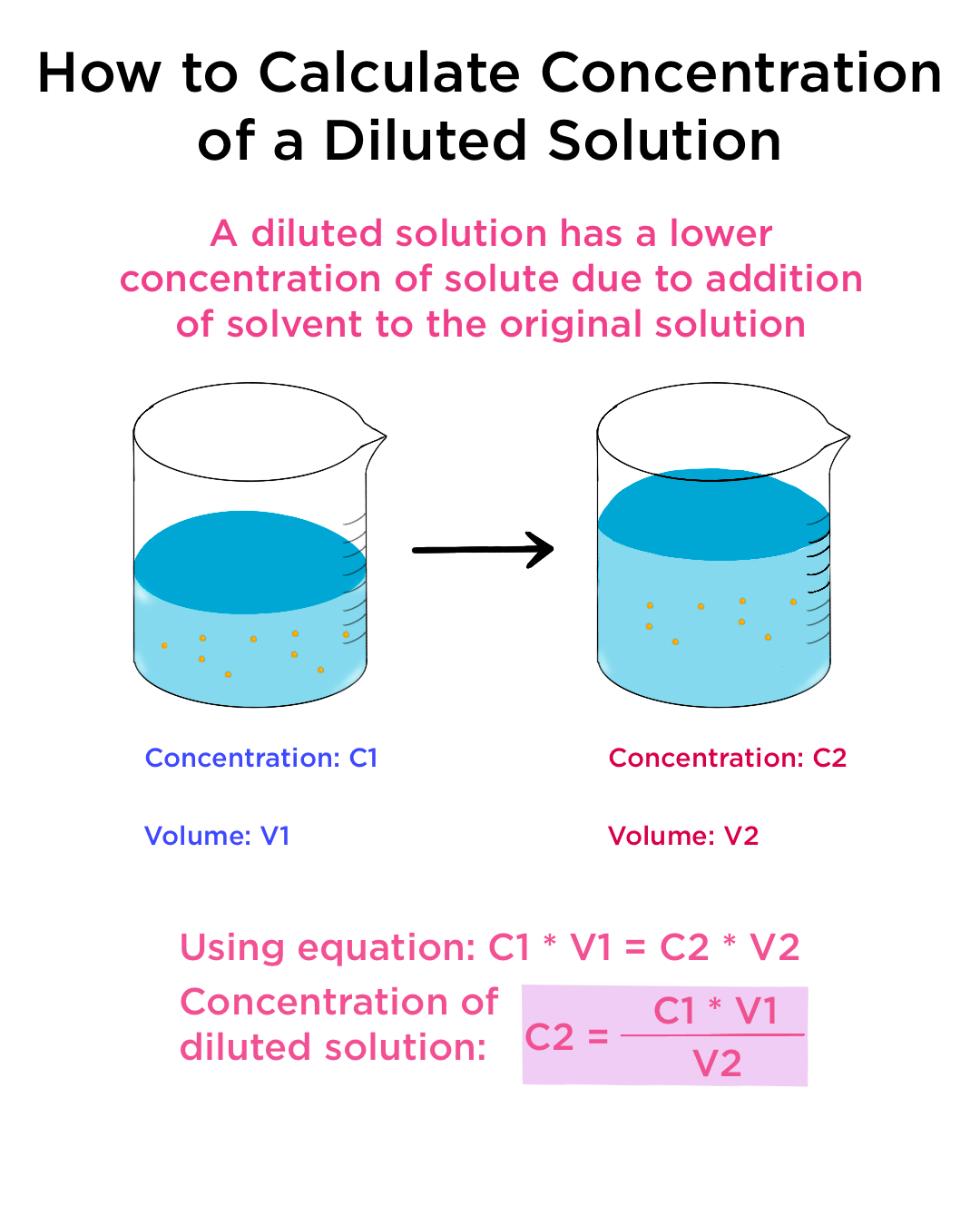
Dilution Calculations Explained . You are simply increasing the amount of solvent in the. The chemist can do it simply by mixing with more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. Understand how stock solutions are used in the laboratory. In any dilution, the number of moles of solute stays the same. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the process of reducing the concentration of a given solute in its solution. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: In this topic, the student will learn and. We are often concerned with. Explain how concentrations can be changed in the lab. C 1 v 1 = c 2 v 2 where:
from www.expii.com
Often, a worker will need to change the concentration of a solution by changing the. Explain how concentrations can be changed in the lab. State whether the concentration of a solution is directly or indirectly proportional to its volume. In this topic, the student will learn and. In any dilution, the number of moles of solute stays the same. The chemist can do it simply by mixing with more solvent. You are simply increasing the amount of solvent in the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Learn how to dilute and concentrate solutions. Dilution is a process used to lower the concentration of the original solution by adding more solvent.
Dilution of Solutions — Overview & Examples Expii
Dilution Calculations Explained The chemist can do it simply by mixing with more solvent. The chemist can do it simply by mixing with more solvent. In this topic, the student will learn and. Explain how concentrations can be changed in the lab. Learn how to dilute and concentrate solutions. Understand how stock solutions are used in the laboratory. Dilution is the process of reducing the concentration of a given solute in its solution. You are simply increasing the amount of solvent in the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is a process used to lower the concentration of the original solution by adding more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. In any dilution, the number of moles of solute stays the same. Often, a worker will need to change the concentration of a solution by changing the. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: We are often concerned with.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Calculations Explained Learn how to dilute and concentrate solutions. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Dilution is a process used to lower the concentration of the original solution by adding more solvent. Explain how concentrations can be changed in the lab. We are often concerned with. The chemist can do. Dilution Calculations Explained.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Dilution Calculations Explained You are simply increasing the amount of solvent in the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. Dilution is the process of reducing the concentration of a given solute in its solution. This video and written tutorial will guide you through how to. Dilution Calculations Explained.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Dilution Calculations Explained In this topic, the student will learn and. The chemist can do it simply by mixing with more solvent. Understand how stock solutions are used in the laboratory. C 1 v 1 = c 2 v 2 where: This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution. Dilution Calculations Explained.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Calculations Explained Understand how stock solutions are used in the laboratory. Often, a worker will need to change the concentration of a solution by changing the. You are simply increasing the amount of solvent in the. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Explain how concentrations can be changed in the. Dilution Calculations Explained.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Calculations Explained State whether the concentration of a solution is directly or indirectly proportional to its volume. In this topic, the student will learn and. In any dilution, the number of moles of solute stays the same. You are simply increasing the amount of solvent in the. Learn how to dilute and concentrate solutions. C 1 v 1 = c 2 v. Dilution Calculations Explained.
From sciencesavers.info
System, Calculator, Methodology, Makes use of, Examples sciencesavers Dilution Calculations Explained Explain how concentrations can be changed in the lab. C 1 v 1 = c 2 v 2 where: The chemist can do it simply by mixing with more solvent. Often, a worker will need to change the concentration of a solution by changing the. Dilution is a process used to lower the concentration of the original solution by adding. Dilution Calculations Explained.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Calculations Explained Understand how stock solutions are used in the laboratory. Explain how concentrations can be changed in the lab. Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the process of reducing the concentration of a given solute. Dilution Calculations Explained.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Calculations Explained This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. You are simply increasing the amount of solvent in the. Explain how concentrations can be changed in the lab. Learn how to dilute and concentrate solutions. The chemist can do it simply by mixing with more solvent. Often, a. Dilution Calculations Explained.
From galaxyopec.weebly.com
How to do dilution series calculations galaxyopec Dilution Calculations Explained The chemist can do it simply by mixing with more solvent. We are often concerned with. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Explain how concentrations can be changed in the lab. You are simply increasing the amount of solvent in the. Understand how stock solutions are used in. Dilution Calculations Explained.
From printablelibbulges.z21.web.core.windows.net
Dilution And Concentration Calculator Dilution Calculations Explained Dilution is the process of reducing the concentration of a given solute in its solution. In any dilution, the number of moles of solute stays the same. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: You are simply increasing the amount of solvent in the. In this topic, the student. Dilution Calculations Explained.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Calculations Explained Explain how concentrations can be changed in the lab. Dilution is the process of reducing the concentration of a given solute in its solution. Understand how stock solutions are used in the laboratory. In this topic, the student will learn and. The chemist can do it simply by mixing with more solvent. Learn how to dilute and concentrate solutions. Dilution. Dilution Calculations Explained.
From www.slideserve.com
PPT Concentration of Solutions PowerPoint Presentation, free download Dilution Calculations Explained Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Explain how concentrations can be changed in the lab. In any dilution, the number of moles of solute stays the same.. Dilution Calculations Explained.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Calculations Explained In any dilution, the number of moles of solute stays the same. We are often concerned with. C 1 v 1 = c 2 v 2 where: Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more solvent. State whether the concentration of a solution is directly or indirectly proportional to. Dilution Calculations Explained.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Calculations Explained To make a fixed amount of a dilute solution from a stock solution, you can use the formula: We are often concerned with. The chemist can do it simply by mixing with more solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. This video and written tutorial will guide you through how to. Dilution Calculations Explained.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Dilution Calculations Explained C 1 v 1 = c 2 v 2 where: Understand how stock solutions are used in the laboratory. State whether the concentration of a solution is directly or indirectly proportional to its volume. In this topic, the student will learn and. This video and written tutorial will guide you through how to make a dilution and how to calculate. Dilution Calculations Explained.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Calculations Explained The chemist can do it simply by mixing with more solvent. In any dilution, the number of moles of solute stays the same. C 1 v 1 = c 2 v 2 where: Explain how concentrations can be changed in the lab. State whether the concentration of a solution is directly or indirectly proportional to its volume. Understand how stock. Dilution Calculations Explained.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Calculations Explained We are often concerned with. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. The chemist can do it simply by mixing with more solvent. You are simply increasing the amount of solvent in the. Explain how concentrations can be changed in the lab. Dilution is the process. Dilution Calculations Explained.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Calculations Explained We are often concerned with. C 1 v 1 = c 2 v 2 where: Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Explain how concentrations can be changed in the lab. Understand how stock solutions are used. Dilution Calculations Explained.
From slideplayer.com
Chapter 8 Solutions 8.5 Molarity and Dilution. ppt download Dilution Calculations Explained Learn how to dilute and concentrate solutions. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Understand how stock solutions are used in the laboratory. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: State whether the concentration of. Dilution Calculations Explained.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Calculations Explained Learn how to dilute and concentrate solutions. Explain how concentrations can be changed in the lab. In this topic, the student will learn and. Often, a worker will need to change the concentration of a solution by changing the. The chemist can do it simply by mixing with more solvent. C 1 v 1 = c 2 v 2 where:. Dilution Calculations Explained.
From www.slideshare.net
Serial dilution Dilution Calculations Explained You are simply increasing the amount of solvent in the. Learn how to dilute and concentrate solutions. The chemist can do it simply by mixing with more solvent. In any dilution, the number of moles of solute stays the same. Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Often, a. Dilution Calculations Explained.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Calculations Explained This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. The chemist can do it simply by mixing with more solvent. You are simply increasing the amount of solvent in the. In this topic, the student will learn and. Understand how stock solutions are used in the laboratory. To. Dilution Calculations Explained.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Calculations Explained In this topic, the student will learn and. The chemist can do it simply by mixing with more solvent. Learn how to dilute and concentrate solutions. You are simply increasing the amount of solvent in the. In any dilution, the number of moles of solute stays the same. Dilution is the process of reducing the concentration of a given solute. Dilution Calculations Explained.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Calculations Explained This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: In any dilution, the number of. Dilution Calculations Explained.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Calculations Explained Explain how concentrations can be changed in the lab. You are simply increasing the amount of solvent in the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. C 1 v 1 = c 2 v 2 where: Understand how stock solutions are used in the laboratory. To make a fixed amount. Dilution Calculations Explained.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Calculations Explained Dilution is the process of reducing the concentration of a given solute in its solution. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. In this topic, the student will learn and. To make a fixed amount of a dilute solution from a stock solution, you can use. Dilution Calculations Explained.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Calculations Explained State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: The chemist can do it simply by mixing with more solvent. You are simply increasing the amount. Dilution Calculations Explained.
From www.davinawellness.com
Dilution Guidelines Dilution Calculations Explained To make a fixed amount of a dilute solution from a stock solution, you can use the formula: State whether the concentration of a solution is directly or indirectly proportional to its volume. In this topic, the student will learn and. Dilution is the process of reducing the concentration of a given solute in its solution. In any dilution, the. Dilution Calculations Explained.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Calculations Explained Dilution is the process of reducing the concentration of a given solute in its solution. Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more solvent. You are simply increasing the amount of solvent in the. State whether the concentration of a solution is directly or indirectly proportional to its volume.. Dilution Calculations Explained.
From www.youtube.com
Solution dilution calculations YouTube Dilution Calculations Explained Understand how stock solutions are used in the laboratory. The chemist can do it simply by mixing with more solvent. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: Explain how concentrations can be changed in the lab. In this topic, the student will learn and. We are often concerned with.. Dilution Calculations Explained.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Calculations Explained The chemist can do it simply by mixing with more solvent. In this topic, the student will learn and. Explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. You are simply increasing the amount of solvent in the. Often, a worker will need to change the concentration of a solution by. Dilution Calculations Explained.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Calculations Explained Dilution is a process used to lower the concentration of the original solution by adding more solvent. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. The chemist can do it simply by mixing with more solvent. State whether the concentration of a solution is directly or indirectly. Dilution Calculations Explained.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Calculations Explained Often, a worker will need to change the concentration of a solution by changing the. Dilution is a process used to lower the concentration of the original solution by adding more solvent. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. The chemist can do it simply. Dilution Calculations Explained.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Calculations Explained Dilution is a process used to lower the concentration of the original solution by adding more solvent. You are simply increasing the amount of solvent in the. To make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: Dilution is the process of. Dilution Calculations Explained.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Calculations Explained To make a fixed amount of a dilute solution from a stock solution, you can use the formula: This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. You are simply increasing the amount of solvent in the. Dilution is a process used to lower the concentration of the. Dilution Calculations Explained.