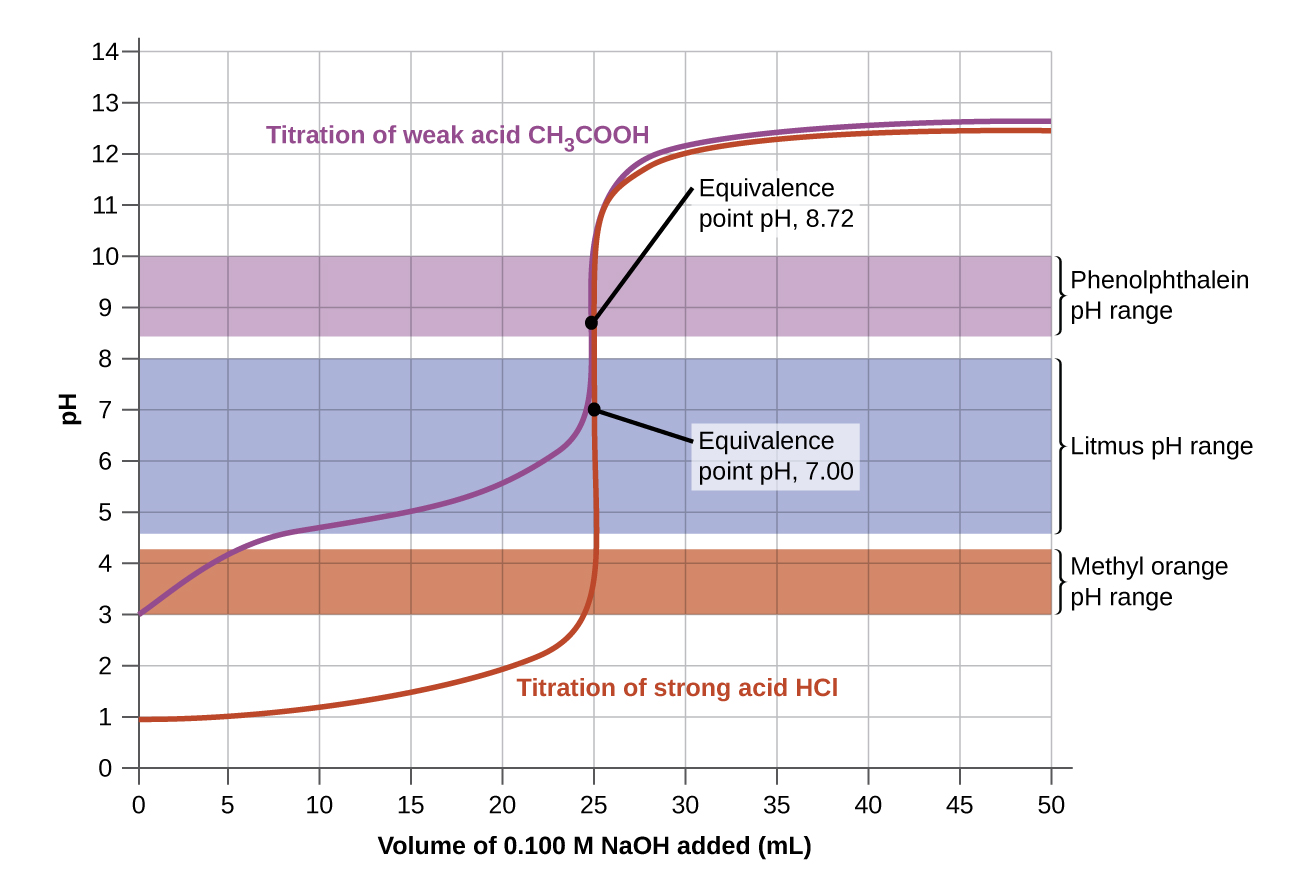
Titration Curve Definition Simple . The titration curve is a plot of changes of a selected property of a solution during titration. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. In the case of alkalimetric titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The property selection depends on the titration: A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. It provides valuable information about the reaction under study and helps. The titration curve is a graphical representation of the ph or other property changes during a titration experiment.
from chem.libretexts.org
A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. The titration curve is a plot of changes of a selected property of a solution during titration. The figure below shows two different examples of a strong. The property selection depends on the titration: Both equivalence points are visible. In the case of alkalimetric titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations
Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. In the case of alkalimetric titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a plot of changes of a selected property of a solution during titration. The property selection depends on the titration: The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. Both equivalence points are visible. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the reaction under study and helps.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Titration Curve Definition Simple The property selection depends on the titration: Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes. Titration Curve Definition Simple.
From mungfali.com
Strong Acid And Base Titration Curve Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. Both equivalence points are visible. The property selection depends on the titration: In the case of alkalimetric titration. The titration curve is a plot of changes of a selected property of a solution during titration. The. Titration Curve Definition Simple.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Definition Simple Both equivalence points are visible. It provides valuable information about the reaction under study and helps. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is. Titration Curve Definition Simple.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Definition Simple The titration curve is a plot of changes of a selected property of a solution during titration. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. Titration curves. Titration Curve Definition Simple.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Definition Simple The titration curve is a plot of changes of a selected property of a solution during titration. The figure below shows two different examples of a strong. The property selection depends on the titration: The end point of a titration is the point at which an indicator changes. The titration curve is a graphical representation of the ph or other. Titration Curve Definition Simple.
From slidetodoc.com
Acid Base Titrations Titration Curve A titration curve Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. The titration curve is a plot of changes of a selected property of a solution during titration. The end point of a titration is the point at which an indicator changes. It provides valuable. Titration Curve Definition Simple.
From giorvlvoc.blob.core.windows.net
Acid Base Titration Curve at Adrian Yount blog Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. The end point of a titration is the point at which an indicator changes. The property selection depends on the titration: In the case of alkalimetric titration. Both equivalence points are visible. The titration. Titration Curve Definition Simple.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A typical titration curve of a diprotic acid, oxalic acid, titrated. Titration Curve Definition Simple.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Definition Simple The property selection depends on the titration: A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve. Titration Curve Definition Simple.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Curve Definition Simple Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The property selection depends on the titration: Both equivalence points are visible. The end point of a titration is the. Titration Curve Definition Simple.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Curve Definition Simple Both equivalence points are visible. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows two different examples of a strong.. Titration Curve Definition Simple.
From chem.libretexts.org
11.4 Reaction Stoichiometry in Solutions OxidationReduction Titration Curve Definition Simple The property selection depends on the titration: The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added. Titration Curve Definition Simple.
From www.hanlin.com
AQA A Level Chemistry复习笔记8.1.9 Titration Curves翰林国际教育 Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. In the case of alkalimetric titration. The titration curve is a plot of changes of a selected property of a solution during titration. The property selection depends on the titration: Both equivalence points are visible. Titration curves show how the ph of an acidic. Titration Curve Definition Simple.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Definition Simple In the case of alkalimetric titration. It provides valuable information about the reaction under study and helps. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a solution during a titration. The property selection depends on the titration: Titration curves show. Titration Curve Definition Simple.
From www.numerade.com
SOLVEDThe graph shows the titration curves for two Titration Curve Definition Simple The titration curve is a plot of changes of a selected property of a solution during titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of a solution during a titration. In the case of. Titration Curve Definition Simple.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Titration Curve Definition Simple It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Both equivalence points are visible. Titration curves show how the ph of an acidic or. Titration Curve Definition Simple.
From www.chegg.com
Solved 24. The figure below shows the titration curves of Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a plot of changes of a selected property of a solution during titration. The figure below shows two different examples of a strong. It provides valuable information about the reaction under study and helps. A typical titration curve of a. Titration Curve Definition Simple.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Definition Simple In the case of alkalimetric titration. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It provides valuable information about the reaction under study and helps. A typical titration curve. Titration Curve Definition Simple.
From www.grace.umd.edu
Simulation of Monoprotic Titration Curve Titration Curve Definition Simple Both equivalence points are visible. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The property selection depends on the titration: In the case of alkalimetric titration. The titration curve is a plot of changes of a selected property of a solution during titration. A titration. Titration Curve Definition Simple.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Definition Simple A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or. Titration Curve Definition Simple.
From 88guru.com
Acid Base Titration What is a Titration Curve? 88guru Titration Curve Definition Simple The property selection depends on the titration: The figure below shows two different examples of a strong. The end point of a titration is the point at which an indicator changes. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a. Titration Curve Definition Simple.
From www.slideserve.com
PPT Amino Acids PowerPoint Presentation, free download ID1842689 Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. The property selection depends on the titration: In the case of alkalimetric titration. The titration. Titration Curve Definition Simple.
From brainly.com
The graphs labeled (a) and (b) show the titration curves for two equal Titration Curve Definition Simple The titration curve is a plot of changes of a selected property of a solution during titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The property selection depends on the titration: The. Titration Curve Definition Simple.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Definition Simple A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In the case of alkalimetric titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The property selection depends on the titration: A titration curve is a graphical representation. Titration Curve Definition Simple.
From mungfali.com
Acid Titration Curve Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The property selection depends on the titration: The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A typical titration curve of. Titration Curve Definition Simple.
From morioh.com
Acid Base Titration Curves PH Calculations Titration Curve Definition Simple The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a plot of changes of a. Titration Curve Definition Simple.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Definition Simple A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below. Titration Curve Definition Simple.
From chem.libretexts.org
11.3 Reaction Stoichiometry in Solutions AcidBase Titrations Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The property selection depends on. Titration Curve Definition Simple.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. In the case of alkalimetric titration. It provides valuable information about the reaction under study and helps. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a plot of changes of a selected property. Titration Curve Definition Simple.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Definition Simple Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. In the case of alkalimetric titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong. The property selection depends. Titration Curve Definition Simple.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Definition Simple Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. In the case of alkalimetric titration. It provides valuable information about the reaction under study and helps. The property selection depends on the titration: The end point of a titration is the point at which an indicator. Titration Curve Definition Simple.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve Definition Simple The titration curve is a plot of changes of a selected property of a solution during titration. It provides valuable information about the reaction under study and helps. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. The titration curve is a graphical representation of. Titration Curve Definition Simple.
From www.numerade.com
SOLVED Match the provided labels to the appropriate point on the Titration Curve Definition Simple The end point of a titration is the point at which an indicator changes. In the case of alkalimetric titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a plot of changes of a selected property of a solution during titration.. Titration Curve Definition Simple.
From www.myxxgirl.com
What Is Double Titration Theory By Using Sulphuric Acid My XXX Hot Girl Titration Curve Definition Simple The property selection depends on the titration: Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a plot of changes of. Titration Curve Definition Simple.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Definition Simple Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. In the case of alkalimetric titration. Titration curves show how the ph of an acidic or basic solution changes as a basic. Titration Curve Definition Simple.