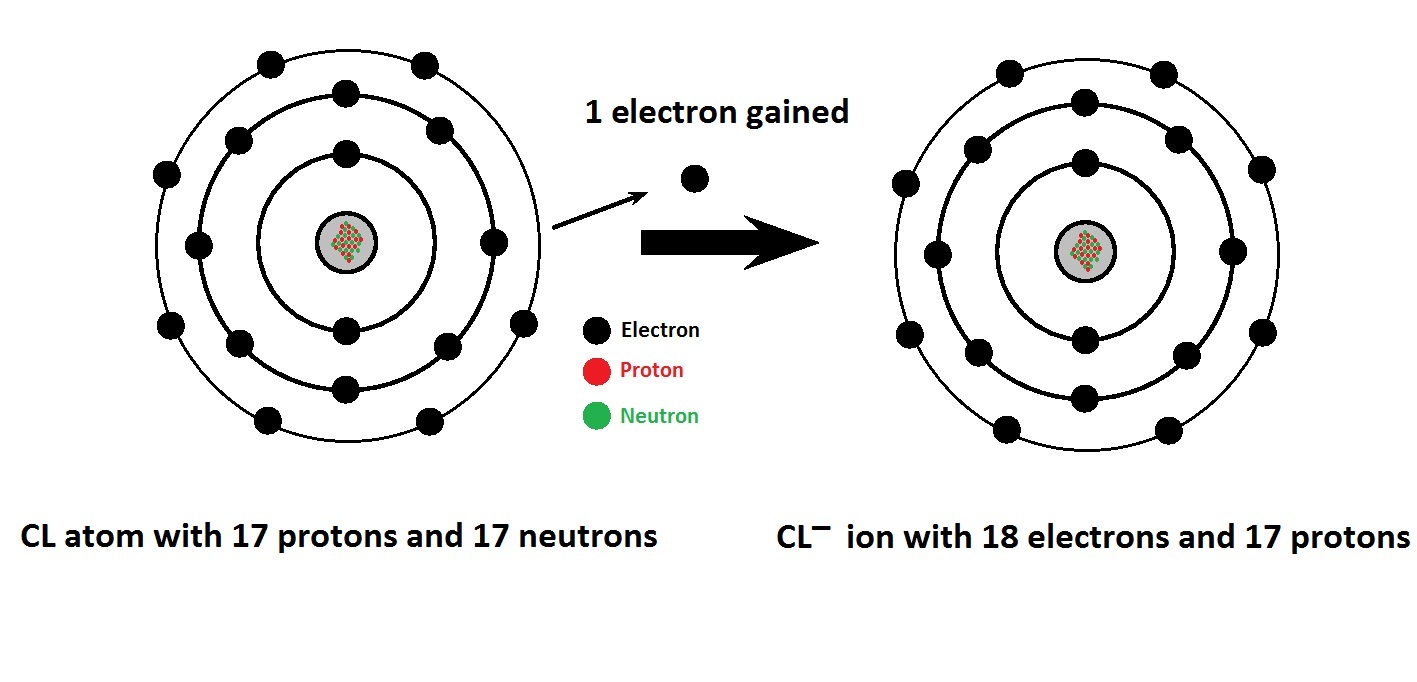
Chlorine Attract Or Release Electrons . Consequences of high electronegativity on the bohr. The electrons are arranged in three energy levels: The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. Two electrons are transferred from the calcium atom, one to each chlorine atom. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. On the right, the chloride ion has 18 electrons and. 2 in the first, 8 in the second, and 7 in the outermost third level.
from basichemistry.blogspot.com
On the right, the chloride ion has 18 electrons and. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. 2 in the first, 8 in the second, and 7 in the outermost third level. Consequences of high electronegativity on the bohr. Two electrons are transferred from the calcium atom, one to each chlorine atom. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. On the left, the chlorine atom has 17 electrons. The formation of a chlorine ion.
Basic Chemistry October 2012
Chlorine Attract Or Release Electrons Two electrons are transferred from the calcium atom, one to each chlorine atom. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Two electrons are transferred from the calcium atom, one to each chlorine atom. On the right, the chloride ion has 18 electrons and. 2 in the first, 8 in the second, and 7 in the outermost third level. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. The formation of a chlorine ion. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The electrons are arranged in three energy levels: On the left, the chlorine atom has 17 electrons. Consequences of high electronegativity on the bohr.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent Chlorine Attract Or Release Electrons The formation of a chlorine ion. Consequences of high electronegativity on the bohr. Two electrons are transferred from the calcium atom, one to each chlorine atom. The electrons are arranged in three energy levels: On the right, the chloride ion has 18 electrons and. 2 in the first, 8 in the second, and 7 in the outermost third level. Since. Chlorine Attract Or Release Electrons.
From slideplayer.com
Ionic Bonds. ppt download Chlorine Attract Or Release Electrons On the right, the chloride ion has 18 electrons and. Two electrons are transferred from the calcium atom, one to each chlorine atom. Consequences of high electronegativity on the bohr. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. When many ions attract each other, they form large,. Chlorine Attract Or Release Electrons.
From sciencenotes.org
Argon Facts Chlorine Attract Or Release Electrons The electrons are arranged in three energy levels: The formation of a chlorine ion. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. On the right, the chloride ion has. Chlorine Attract Or Release Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. On the left, the chlorine atom has 17 electrons. Two electrons are transferred from the calcium atom, one to each chlorine atom. When many ions attract each other, they form large, ordered, crystal lattices in which each. Chlorine Attract Or Release Electrons.
From slideplayer.com
You don’t have to go to the ends of the earth to find POLAR MOLECULES Chlorine Attract Or Release Electrons The formation of a chlorine ion. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. When many ions attract each other, they form large, ordered, crystal lattices in which each. Chlorine Attract Or Release Electrons.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Attract Or Release Electrons The electrons are arranged in three energy levels: The formation of a chlorine ion. 2 in the first, 8 in the second, and 7 in the outermost third level. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. On the right, the chloride ion has 18 electrons and.. Chlorine Attract Or Release Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Attract Or Release Electrons On the right, the chloride ion has 18 electrons and. Two electrons are transferred from the calcium atom, one to each chlorine atom. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The first electron affinity is the energy released when 1 mole of gaseous. Chlorine Attract Or Release Electrons.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Attract Or Release Electrons On the left, the chlorine atom has 17 electrons. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Consequences of high electronegativity on the bohr. Two electrons are transferred from the calcium atom, one to each chlorine atom. 2 in the first, 8 in the second, and 7 in the outermost. Chlorine Attract Or Release Electrons.
From askfilo.com
Although chlorine is an electron withdrawing group, yet it is ortho, par.. Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to. Chlorine Attract Or Release Electrons.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Attract Or Release Electrons The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. On the left, the chlorine atom has 17 electrons. The electrons are arranged in three energy levels: On the right, the chloride ion has 18 electrons and. 2 in the first, 8 in the second, and 7 in the outermost third level.. Chlorine Attract Or Release Electrons.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The electrons are arranged in three energy levels: Consequences of high electronegativity on the bohr. The formation of a chlorine ion. On the right, the chloride ion has 18 electrons and. The electronegativity, represented by the pauling. Chlorine Attract Or Release Electrons.
From www.chegg.com
Solved Does potassium (K) attract or release electrons? How Chlorine Attract Or Release Electrons The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Two electrons are transferred from the calcium atom, one to each chlorine atom. The formation of a chlorine ion.. Chlorine Attract Or Release Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Attract Or Release Electrons On the right, the chloride ion has 18 electrons and. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. The electrons are arranged in three energy levels: On the left, the chlorine atom has 17 electrons. 2 in the first, 8 in the second, and 7 in the outermost third level.. Chlorine Attract Or Release Electrons.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I Chlorine Attract Or Release Electrons The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. 2 in the first, 8 in the second, and 7 in the outermost third level. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The electrons are arranged in three. Chlorine Attract Or Release Electrons.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Attract Or Release Electrons Consequences of high electronegativity on the bohr. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. When many ions attract each other, they form large, ordered, crystal lattices in which. Chlorine Attract Or Release Electrons.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Attract Or Release Electrons On the right, the chloride ion has 18 electrons and. On the left, the chlorine atom has 17 electrons. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. 2 in the first, 8 in the second, and 7 in the outermost third level. Consequences of high electronegativity on the bohr. The. Chlorine Attract Or Release Electrons.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The formation of a chlorine ion. Consequences of high electronegativity on the bohr. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. The electrons are. Chlorine Attract Or Release Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Attract Or Release Electrons The electrons are arranged in three energy levels: Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. On the left, the chlorine atom has. Chlorine Attract Or Release Electrons.
From www.numerade.com
SOLVED Chlorine atoms and iodine atoms can both gain electron to form Chlorine Attract Or Release Electrons The electrons are arranged in three energy levels: 2 in the first, 8 in the second, and 7 in the outermost third level. Consequences of high electronegativity on the bohr. On the right, the chloride ion has 18 electrons and. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions. Chlorine Attract Or Release Electrons.
From sestbio.com
Chlorine Dioxide Fast/Slow Release Sachet Chlorine Dioxide Tablets Chlorine Attract Or Release Electrons On the right, the chloride ion has 18 electrons and. Two electrons are transferred from the calcium atom, one to each chlorine atom. 2 in the first, 8 in the second, and 7 in the outermost third level. The electrons are arranged in three energy levels: When many ions attract each other, they form large, ordered, crystal lattices in which. Chlorine Attract Or Release Electrons.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Attract Or Release Electrons Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. On the right, the chloride ion has 18 electrons and. The electrons are arranged in three energy levels: 2 in the first, 8 in the second, and 7 in the outermost third level. Two electrons are transferred from the. Chlorine Attract Or Release Electrons.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Attract Or Release Electrons 2 in the first, 8 in the second, and 7 in the outermost third level. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Consequences of high electronegativity. Chlorine Attract Or Release Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Chlorine Have? Chlorine Attract Or Release Electrons Two electrons are transferred from the calcium atom, one to each chlorine atom. The formation of a chlorine ion. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. On the left, the chlorine atom has 17 electrons. Since fluorine has its valence electrons in the n=2. Chlorine Attract Or Release Electrons.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Attract Or Release Electrons Two electrons are transferred from the calcium atom, one to each chlorine atom. The formation of a chlorine ion. When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract. Chlorine Attract Or Release Electrons.
From basichemistry.blogspot.com
Basic Chemistry October 2012 Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Two electrons are transferred from the calcium atom, one to each chlorine atom. The electrons are arranged in three energy levels: When many ions attract each other, they form large, ordered, crystal lattices in which each ion. Chlorine Attract Or Release Electrons.
From brainly.com
Use the images to explain why carbon forms a bond with four chlorine Chlorine Attract Or Release Electrons Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. Consequences of high electronegativity on the bohr. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The electrons are arranged in three energy levels: 2. Chlorine Attract Or Release Electrons.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Attract Or Release Electrons On the left, the chlorine atom has 17 electrons. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Two electrons are transferred from the calcium atom, one to each chlorine atom. Consequences of high electronegativity on the bohr. The electronegativity, represented by the pauling scale, is. Chlorine Attract Or Release Electrons.
From socratic.org
Chlorine combined with two negative atom or 1 positive and other Chlorine Attract Or Release Electrons The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. On the right, the chloride ion has 18 electrons and. On the left, the chlorine atom has 17 electrons. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons. Chlorine Attract Or Release Electrons.
From www.numerade.com
When chlorine gains an electron to a chloride ion with a 21 Chlorine Attract Or Release Electrons When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. 2 in the first, 8 in the second, and 7 in the outermost third level.. Chlorine Attract Or Release Electrons.
From slideplayer.com
Write the electron configuration for these elements… ppt download Chlorine Attract Or Release Electrons On the left, the chlorine atom has 17 electrons. The electrons are arranged in three energy levels: Since fluorine has its valence electrons in the n=2 energy level, and since chlorine has its valence electrons in the n=3. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. The formation of a. Chlorine Attract Or Release Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Attract Or Release Electrons The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. Two electrons are transferred from the calcium atom, one to each chlorine atom. The formation of a chlorine ion. Consequences of high electronegativity on the bohr. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire. Chlorine Attract Or Release Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Attract Or Release Electrons 2 in the first, 8 in the second, and 7 in the outermost third level. The formation of a chlorine ion. Consequences of high electronegativity on the bohr. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Two electrons are transferred from the calcium atom, one. Chlorine Attract Or Release Electrons.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Attract Or Release Electrons Consequences of high electronegativity on the bohr. The electrons are arranged in three energy levels: The formation of a chlorine ion. 2 in the first, 8 in the second, and 7 in the outermost third level. The electronegativity, represented by the pauling scale, is 3.16 for chlorine, indicating its strong tendency to attract electrons. When many ions attract each other,. Chlorine Attract Or Release Electrons.
From saylordotorg.github.io
Ionic Bonding and Simple Ionic Compounds Chlorine Attract Or Release Electrons When many ions attract each other, they form large, ordered, crystal lattices in which each ion is surrounded by ions of the opposite charge. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. Two electrons are transferred from the calcium atom, one to each chlorine atom.. Chlorine Attract Or Release Electrons.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Attract Or Release Electrons 2 in the first, 8 in the second, and 7 in the outermost third level. Two electrons are transferred from the calcium atom, one to each chlorine atom. The electrons are arranged in three energy levels: The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of. The. Chlorine Attract Or Release Electrons.