Iron Nuclear Charge . For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: We denote it by z_\text {eff} z eff. Z_\text {eff} = z z eff = z. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Zeff = z − s (7.2.1). Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2:
from www.frontiersin.org
Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: We denote it by z_\text {eff} z eff. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Z_\text {eff} = z z eff = z. Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Zeff = z − s (7.2.1).
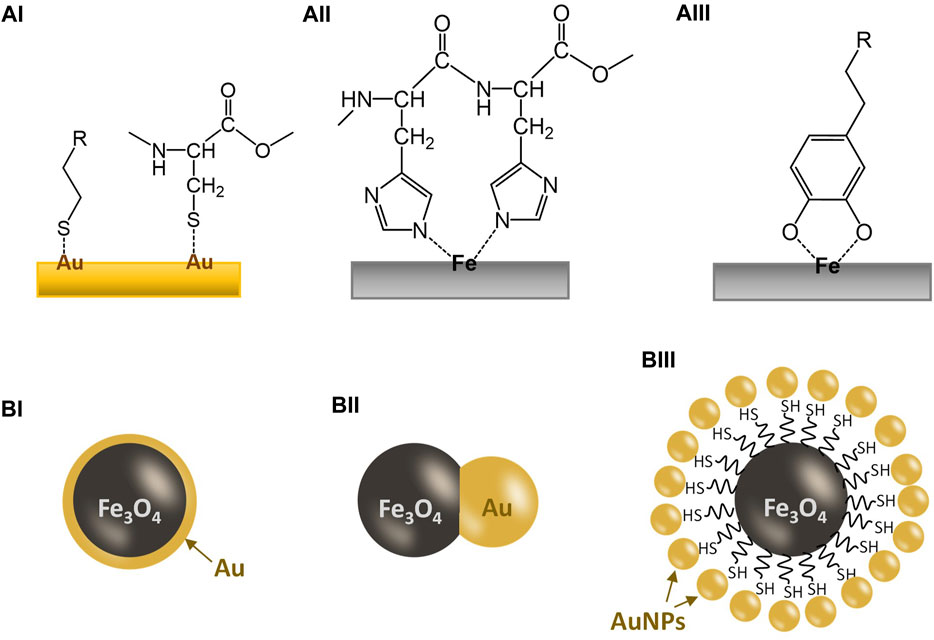
Frontiers Goldiron oxide (Au/Fe3O4) nanoparticles as the
Iron Nuclear Charge We denote it by z_\text {eff} z eff. We denote it by z_\text {eff} z eff. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Zeff = z − s (7.2.1). Z_\text {eff} = z z eff = z.
From www.frontiersin.org
Frontiers Goldiron oxide (Au/Fe3O4) nanoparticles as the Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\). Iron Nuclear Charge.
From manuallistgalumphed.z21.web.core.windows.net
Electronic Configuration For Gallium Iron Nuclear Charge Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Z_\text {eff} = z z eff = z. Zeff = z − s (7.2.1). We denote it by z_\text {eff} z eff. The amount of positive nuclear charge. Iron Nuclear Charge.
From slideplayer.com
Periodic Trends. ppt download Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: We denote it by z_\text {eff} z eff. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus. Iron Nuclear Charge.
From brokeasshome.com
How To Find Nuclear Charge On Periodic Table Iron Nuclear Charge Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation:. Iron Nuclear Charge.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Z_\text {eff} = z z eff = z. Zeff = z − s (7.2.1). We denote. Iron Nuclear Charge.
From slideplayer.com
Trends & the Periodic Table ppt download Iron Nuclear Charge Zeff = z − s (7.2.1). Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective nuclear charge. Iron Nuclear Charge.
From www.numerade.com
SOLVEDWhat is effective nuclear charge? What is shielding? Iron Nuclear Charge We denote it by z_\text {eff} z eff. For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and. Iron Nuclear Charge.
From www.sciencelaws.in
√ Science class 10 notes Nuclear reaction full detailed explain Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Z_\text {eff} = z z eff = z. For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective. Iron Nuclear Charge.
From facts.net
11 Captivating Facts About Effective Nuclear Charge Iron Nuclear Charge Z_\text {eff} = z z eff = z. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff = z − s (7.2.1). Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. For. Iron Nuclear Charge.
From www.toppr.com
The mass number of iron nucleus is 56, the nuclear density is Iron Nuclear Charge Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2:. Iron Nuclear Charge.
From slideplayer.com
Trends & the Periodic Table ppt download Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Z_\text {eff} = z z eff = z. Zeff z e f f can be calculated by subtracting the magnitude. Iron Nuclear Charge.
From slideplayer.com
Figure Figure Title Variations in effective nuclear charge. Caption Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The amount of positive nuclear. Iron Nuclear Charge.
From slideplayer.com
The Periodic Law Atoms with similar properties appear in groups or Iron Nuclear Charge Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Z_\text {eff} = z z eff = z. For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: The amount of positive nuclear charge experienced by any. Iron Nuclear Charge.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: We denote it by z_\text {eff} z eff. Its electron configuration is, (1s. Iron Nuclear Charge.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Iron Nuclear Charge Zeff = z − s (7.2.1). Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Zeff z e f f can. Iron Nuclear Charge.
From saylordotorg.github.io
Nuclear Reactions Iron Nuclear Charge Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). We denote it by z_\text {eff} z eff. Slater's rules allow. Iron Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Atomic Energies and Periodicity PowerPoint Presentation Iron Nuclear Charge Zeff = z − s (7.2.1). Z_\text {eff} = z z eff = z. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. We denote it by z_\text {eff} z eff. The amount of positive nuclear charge experienced by any individual. Iron Nuclear Charge.
From www.doubtnut.com
Nuclear charge actually experienced by an electron is termed as effect Iron Nuclear Charge Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Zeff z e f. Iron Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Z_\text {eff} = z z eff = z. For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus. Iron Nuclear Charge.
From slideplayer.com
PERIODIC TRENDS. ppt download Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Zeff = z − s (7.2.1). Zeff z e f f can be calculated by. Iron Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. We denote it by z_\text {eff} z eff. Zeff z e f f can be calculated by subtracting the magnitude. Iron Nuclear Charge.
From brokeasshome.com
How To Find Nuclear Charge On Periodic Table Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). We denote it by z_\text {eff} z eff. Z_\text {eff} = z z eff = z. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in. Iron Nuclear Charge.
From mydigitalkemistry.com
period 3 effective nuclear charge trend Best Online Free Chemistry Iron Nuclear Charge The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). We denote it by z_\text {eff} z eff. Zeff = z − s (7.2.1). Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons. Iron Nuclear Charge.
From www.bartleby.com
Answered Rank the effective nuclear charge Z*… bartleby Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*).. Iron Nuclear Charge.
From slideplayer.com
New topic The Periodic Table ppt download Iron Nuclear Charge We denote it by z_\text {eff} z eff. Zeff = z − s (7.2.1). The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Z_\text {eff} = z z eff = z. Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Slater's rules allow you to estimate the effective nuclear. Iron Nuclear Charge.
From www.nuclear-power.com
Iron Electron Affinity Electronegativity Ionization Energy of Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: We denote it by z_\text {eff} z eff. For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of. Iron Nuclear Charge.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Iron Nuclear Charge Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Z_\text {eff} = z z eff = z. We denote it by z_\text {eff} z eff. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from. Iron Nuclear Charge.
From www.slideserve.com
PPT Chemistry periodicity Atomic and Ionic Radius PowerPoint Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Z_\text {eff} = z z eff = z. The amount of positive nuclear charge experienced by. Iron Nuclear Charge.
From piratarmy.com
Effective Nuclear Charge Of Oxygen Iron Nuclear Charge We denote it by z_\text {eff} z eff. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus and the effective shielding of electrons in each. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff = z − s (7.2.1). Zeff. Iron Nuclear Charge.
From www.slideserve.com
PPT Some more Regents Chemistry practice… PowerPoint Presentation Iron Nuclear Charge Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: We denote it by z_\text {eff} z eff. Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: The amount of positive nuclear charge experienced by. Iron Nuclear Charge.
From slideplayer.com
Periodic trends. ppt download Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Z_\text {eff} = z z eff = z. Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Slater's rules allow you to. Iron Nuclear Charge.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Iron Nuclear Charge We denote it by z_\text {eff} z eff. Zeff = z − s (7.2.1). Its electron configuration is, (1s 2) (2s 2, 2p 6) step 2: The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (z*). Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total. Iron Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Iron Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Z_\text {eff} = z z eff = z. The amount of positive nuclear. Iron Nuclear Charge.
From nanohub.org
Courses PHYS 342 Modern Physics Public SelfPaced Iron Nuclear Charge Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: For the first electron around the nucleus, the effective nuclear charge equals the nuclear charge: Z_\text {eff} = z z eff = z. We denote it by z_\text. Iron Nuclear Charge.
From www.pearson.com
Which experience a greater effective nuclear charge the valence Iron Nuclear Charge Zeff z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation: Z_\text {eff} = z z eff = z. Slater's rules allow you to estimate the effective nuclear charge \(z_{eff}\) from the real number of protons in the nucleus. Iron Nuclear Charge.