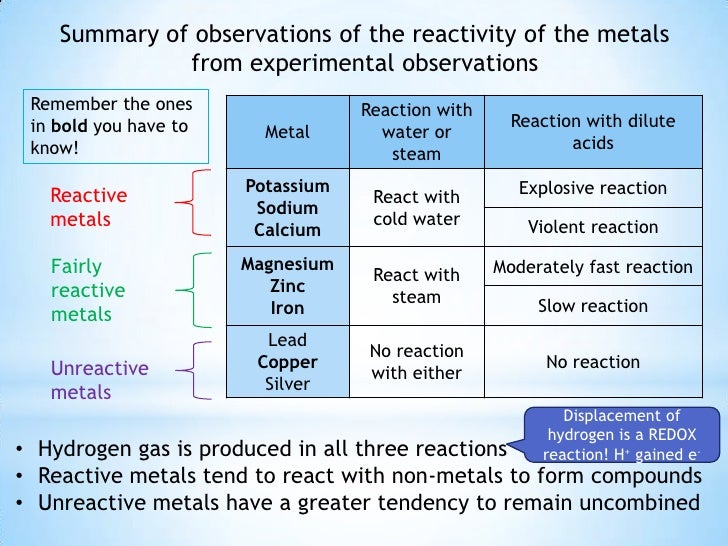
Metal React With Hot Water . Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. The formation of the ions from the original metal. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. Cold water and the metals sodium,. In this case, however, hydrogen gas is produced with the metal. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. However, alkali metals react vigorously with. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water ⇨ metal hydroxide + hydrogen.
from chemistrymsq10.blogspot.com
The formation of the ions from the original metal. In this case, however, hydrogen gas is produced with the metal. The metals below hydrogen in the reactivity series don't react with steam. However, alkali metals react vigorously with. Most of the metals do not react with water. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. Metal + water ⇨ metal hydroxide + hydrogen. Cold water and the metals sodium,. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid.
Grade10 CHAPTER 2 REACTIVITY SERIES SEMESTER 1
Metal React With Hot Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. The metals below hydrogen in the reactivity series don't react with steam. When group 2 metals react to form oxides or hydroxides, metal ions are formed. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Most of the metals do not react with water. Cold water and the metals sodium,. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. In this case, however, hydrogen gas is produced with the metal. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. However, alkali metals react vigorously with. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. The formation of the ions from the original metal. Metal + water ⇨ metal hydroxide + hydrogen.
From www.hanlin.com
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with Metal React With Hot Water However, alkali metals react vigorously with. Cold water and the metals sodium,. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. When group 2 metals react to form oxides or. Metal React With Hot Water.
From ppt-online.org
Metals презентация онлайн Metal React With Hot Water Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. In this case, however, hydrogen gas is produced with the metal. When group 2 metals react to form oxides or hydroxides,. Metal React With Hot Water.
From www.slideshare.net
Summary of Metal Reactions Metal React With Hot Water Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Cold water and the metals sodium,. In this case, however, hydrogen gas is produced with the metal. Metal + water ⇨ metal hydroxide + hydrogen. Most of the metals do not react with water. The formation of the ions from the original. Metal React With Hot Water.
From www.topperlearning.com
The action of all metals on water? 573fusqq Metal React With Hot Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Metal + water ⇨ metal hydroxide + hydrogen. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Cold. Metal React With Hot Water.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Metal React With Hot Water Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. When group 2 metals react to form oxides or hydroxides, metal ions are formed. In this case, however, hydrogen gas is. Metal React With Hot Water.
From edurev.in
Overview (Part 2) Metals And Nonmetals Notes EduRev Metal React With Hot Water Cold water and the metals sodium,. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water.. Metal React With Hot Water.
From www.pinterest.com
Alkali metals react with water to produce hydroxides. Alkali metal Metal React With Hot Water Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Most of the. Metal React With Hot Water.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID5525228 Metal React With Hot Water When group 2 metals react to form oxides or hydroxides, metal ions are formed. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Metal + water ⇨ metal hydroxide + hydrogen. The metals below hydrogen in the reactivity series don't react with steam. However, alkali metals react vigorously with. Most of. Metal React With Hot Water.
From ppt-online.org
The alkali metals презентация онлайн Metal React With Hot Water When group 2 metals react to form oxides or hydroxides, metal ions are formed. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Most of the metals do not react with water. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Metal. Metal React With Hot Water.
From www.slideshare.net
Metals Reactivity Series Metal React With Hot Water Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water ⇨ metal hydroxide. Metal React With Hot Water.
From www.doubtnut.com
Apparatus Beakers. Chemicals Samples of various metals , water. Metal React With Hot Water Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Most of the metals do not react with water. Cold water and the metals sodium,. Similarly to the group 1 oxides, the hydrides of the group 1 elements. Metal React With Hot Water.
From www.bbc.co.uk
BBC Two Bitesize Chemistry, Alkali metals and their reactions to air Metal React With Hot Water The formation of the ions from the original metal. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Cold water and the metals sodium,. Most of the metals do not react with water. Copper(cu) reaction with oxygen (when heated and. Metal React With Hot Water.
From ar.inspiredpencil.com
Metal And Water Reaction Metal React With Hot Water However, alkali metals react vigorously with. In this case, however, hydrogen gas is produced with the metal. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Metal + water = metal oxide + hydrogen is the fundamental. Metal React With Hot Water.
From www.youtube.com
Reaction of metals with steam YouTube Metal React With Hot Water However, alkali metals react vigorously with. When group 2 metals react to form oxides or hydroxides, metal ions are formed. In this case, however, hydrogen gas is produced with the metal. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Similarly to the group 1 oxides, the hydrides of the group 1 elements react. Metal React With Hot Water.
From spmchemistry.blog.onlinetuition.com.my
Reaction of Alkali Metals with Chlorine SPM Chemistry Metal React With Hot Water Metal + water ⇨ metal hydroxide + hydrogen. Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. However, alkali metals react vigorously with. When group 2 metals react to form. Metal React With Hot Water.
From winnerseducation.com
Reaction of Metals with water, steam and dilute acid (1) Winners Metal React With Hot Water The metals below hydrogen in the reactivity series don't react with steam. In this case, however, hydrogen gas is produced with the metal. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. The formation of the ions from the original metal. However, alkali metals react vigorously with. All. Metal React With Hot Water.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Metal React With Hot Water Metal + water ⇨ metal hydroxide + hydrogen. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. When group 2 metals react to form oxides or hydroxides, metal ions are formed. The metals. Metal React With Hot Water.
From www.youtube.com
Reaction of Metals with Cold Water, Chemistry Lecture Sabaq.pk Metal React With Hot Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. The formation of the ions from the original metal. The metals below hydrogen in the reactivity series don't react with steam. Cold water and the metals sodium,. Most of the metals do not react with water. However, alkali metals react vigorously with. Similarly to the. Metal React With Hot Water.
From chemistrymsq10.blogspot.com
Grade10 CHAPTER 2 REACTIVITY SERIES SEMESTER 1 Metal React With Hot Water However, alkali metals react vigorously with. The metals below hydrogen in the reactivity series don't react with steam. Most of the metals do not react with water. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. Cold water and the metals sodium,. All of group 1 elements —lithium, sodium, potassium, rubidium. Metal React With Hot Water.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Metal React With Hot Water The formation of the ions from the original metal. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Cold water and the metals sodium,. Metal + water = metal oxide + hydrogen is the fundamental equation. Metal React With Hot Water.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Metal React With Hot Water Most of the metals do not react with water. Cold water and the metals sodium,. In this case, however, hydrogen gas is produced with the metal. When group 2 metals react to form oxides or hydroxides, metal ions are formed. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water ⇨ metal. Metal React With Hot Water.
From www.thesciencehive.co.uk
The Periodic Table (AQA) — the science hive Metal React With Hot Water Most of the metals do not react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. In this case, however, hydrogen gas is produced with the metal. Metal + water ⇨ metal hydroxide + hydrogen. The formation of the ions from the original metal. Similarly to the group 1 oxides, the hydrides. Metal React With Hot Water.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Metal React With Hot Water Most of the metals do not react with water. Cold water and the metals sodium,. Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Metal + water ⇨ metal hydroxide + hydrogen. However, alkali metals react. Metal React With Hot Water.
From www.youtube.com
Reactions Of Metals With Water Reactions Chemistry FuseSchool Metal React With Hot Water Cold water and the metals sodium,. The formation of the ions from the original metal. Metal + water ⇨ metal hydroxide + hydrogen. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Most. Metal React With Hot Water.
From brainly.in
Compare in tabular form of the reactivities of the following metals Metal React With Hot Water Metal + water ⇨ metal hydroxide + hydrogen. Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Similarly to the group 1 oxides, the hydrides of the group 1 elements. Metal React With Hot Water.
From www.youtube.com
Metals reacting with water Metals and Non metals Chemistry Khan Metal React With Hot Water However, alkali metals react vigorously with. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Similarly to the group 1 oxides, the. Metal React With Hot Water.
From www.youtube.com
Reaction of metal with steam, acid and water YouTube Metal React With Hot Water The formation of the ions from the original metal. The metals below hydrogen in the reactivity series don't react with steam. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. All of group 1. Metal React With Hot Water.
From ar.inspiredpencil.com
Metal And Water Reaction Metal React With Hot Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. The formation of the ions from the original metal. However, alkali metals react vigorously with. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. When group 2 metals react to form oxides or hydroxides, metal ions. Metal React With Hot Water.
From www.youtube.com
O Level Pure Chemistry. IP Chemistry Reaction of Metals with Water Metal React With Hot Water In this case, however, hydrogen gas is produced with the metal. When group 2 metals react to form oxides or hydroxides, metal ions are formed. Most of the metals do not react with water. The formation of the ions from the original metal. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black. Metal React With Hot Water.
From www.slideshare.net
Metals Metal React With Hot Water Metal + water = metal oxide + hydrogen is the fundamental equation for the metal reaction with water. The formation of the ions from the original metal. Most of the metals do not react with water. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. When group 2 metals react to form oxides or. Metal React With Hot Water.
From www.youtube.com
How do metals react with water/Reaction of metals with waterMetals and Metal React With Hot Water In this case, however, hydrogen gas is produced with the metal. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. The formation of the ions from the original metal. Metal + water ⇨ metal hydroxide + hydrogen. Similarly to the group 1 oxides, the hydrides of the group 1 elements react. Metal React With Hot Water.
From byjus.com
Which metal reacts with cold water? Metal React With Hot Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. When group 2 metals react to form oxides or hydroxides, metal ions are formed. The formation of the ions from the original metal. Copper(cu) reaction with oxygen (when heated and at room temperature) reacts on heating to form a black solid. Metal + water ⇨. Metal React With Hot Water.
From www.onlinemathlearning.com
Reaction of Metals (examples, answers, activities, experiment, videos) Metal React With Hot Water When group 2 metals react to form oxides or hydroxides, metal ions are formed. The metals below hydrogen in the reactivity series don't react with steam. Metal + water ⇨ metal hydroxide + hydrogen. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. The formation of the ions. Metal React With Hot Water.
From mammothmemory.net
The reactivity series is a list of common metal elements Metal React With Hot Water Most of the metals do not react with water. The metals below hydrogen in the reactivity series don't react with steam. In this case, however, hydrogen gas is produced with the metal. Cold water and the metals sodium,. Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. All. Metal React With Hot Water.
From www.youtube.com
Calculating the Specific Heat of a Hot Piece of Metal Dropped into Metal React With Hot Water Similarly to the group 1 oxides, the hydrides of the group 1 elements react with water to form a basic solution. The metals below hydrogen in the reactivity series don't react with steam. In this case, however, hydrogen gas is produced with the metal. The formation of the ions from the original metal. Metal + water ⇨ metal hydroxide +. Metal React With Hot Water.