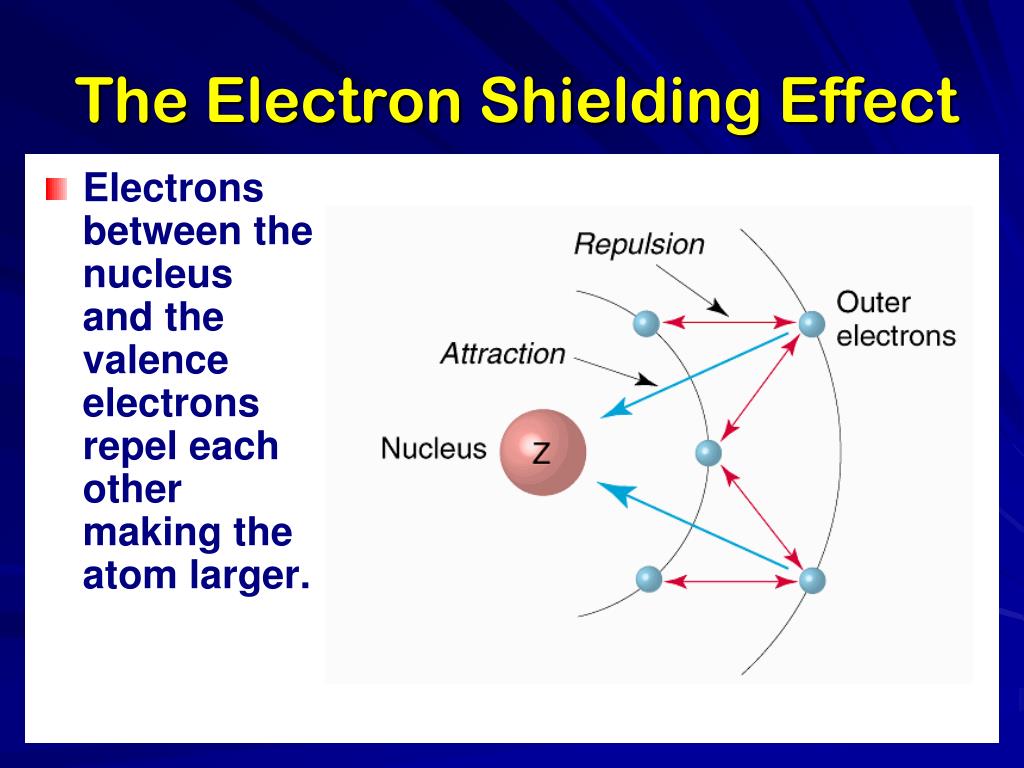
What Is Meant By The Term Shielding In A Many Electron Atom . The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
from www.slideserve.com
The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.
PPT Periodic Table PowerPoint Presentation, free download ID1951146
What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
From www.animalia-life.club
Electron Shell Diagram What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From ar.inspiredpencil.com
Electron Shielding What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID6734591 What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.animalia-life.club
Electron Shell Diagram What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.breakingatom.com
Shielding of the Nucleus What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. Electrons in an \(s\) orbital can shield \(p\) electrons at the. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.numerade.com
SOLVED What is meant by the term "shielding of electrons" in an atom What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation ID3646738 What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.youtube.com
Electron shielding YouTube What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in. What Is Meant By The Term Shielding In A Many Electron Atom.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer.. What Is Meant By The Term Shielding In A Many Electron Atom.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. However, due to the repulsive forces from the inner electrons, the. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From klajgdeuu.blob.core.windows.net
Which Type Of Electrons Are Best At Shielding A 3P Electron at Tyra What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.. What Is Meant By The Term Shielding In A Many Electron Atom.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT Notes One Unit Nine Chapter Four PowerPoint Presentation, free What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Is Meant By The Term Shielding In A Many Electron Atom The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is.. What Is Meant By The Term Shielding In A Many Electron Atom.
From dxoegqbbm.blob.core.windows.net
Electron Shielding Effect Ionization Energy at Elizabeth Shank blog What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. Electrons in an \(s\) orbital can shield \(p\) electrons at the. What Is Meant By The Term Shielding In A Many Electron Atom.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From skynotes.steveyan.com
Introduction to Atoms Nucleus, Protons, Neutrons, Atomic Numbers What Is Meant By The Term Shielding In A Many Electron Atom The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in the force of attraction exerted by the nucleus on the valence. What Is Meant By The Term Shielding In A Many Electron Atom.
From klatysxav.blob.core.windows.net
What Is Electron Shell Configuration at Louis Rozier blog What Is Meant By The Term Shielding In A Many Electron Atom However, due to the repulsive forces from the inner electrons, the. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The shielding effect refers to the phenomenon where inner electrons in an atom repel outer electrons, reducing the effective nuclear charge that outer. The decrease in. What Is Meant By The Term Shielding In A Many Electron Atom.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Is Meant By The Term Shielding In A Many Electron Atom Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is. However, due to the repulsive forces from the inner electrons, the.. What Is Meant By The Term Shielding In A Many Electron Atom.