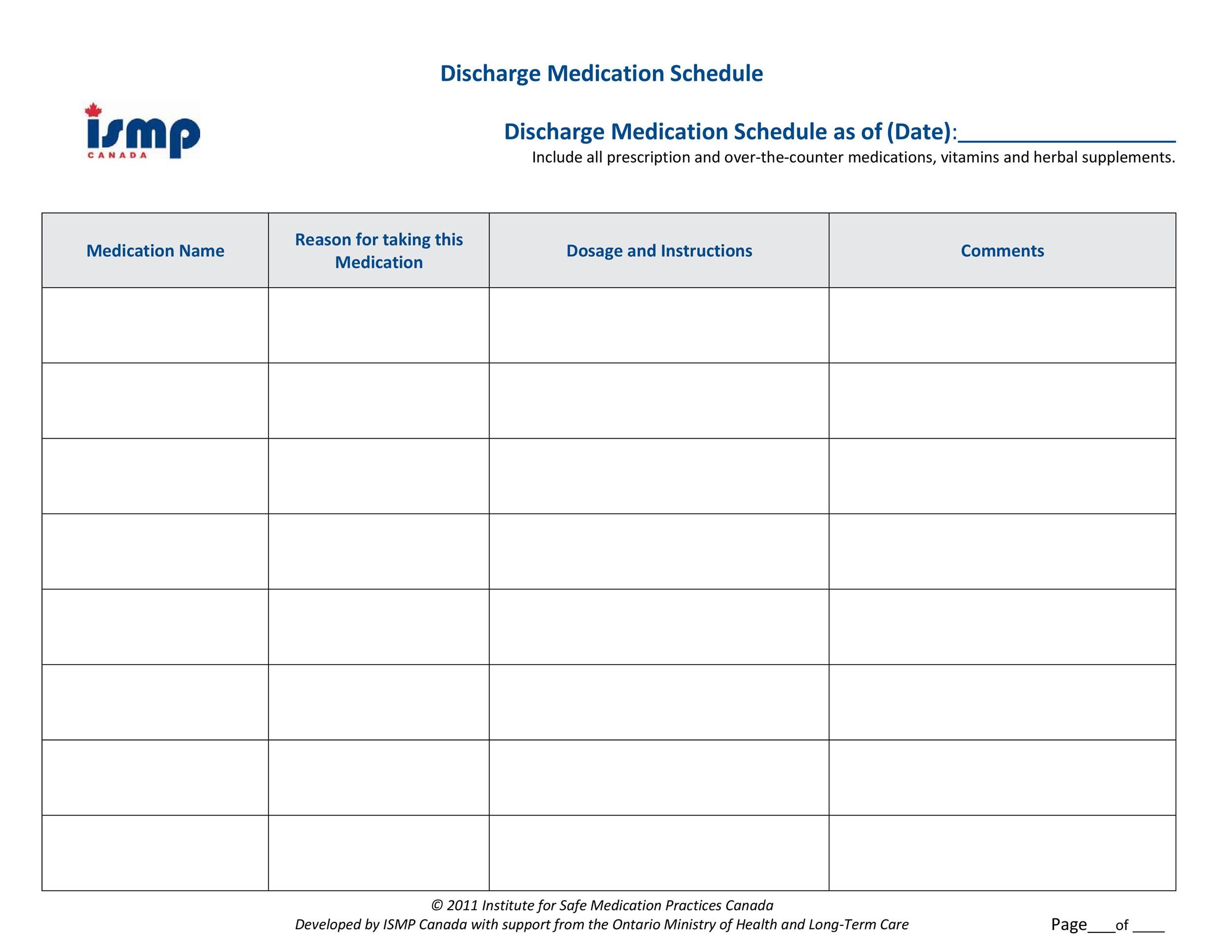
Schedule C Medications . The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule c drugs are radiopharmaceuticals, kits, and generators. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. They are listed on schedule c to the food and drugs.
from templatelab.com
Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. They are listed on schedule c to the food and drugs. Schedule c drugs are radiopharmaceuticals, kits, and generators. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more.
40 Great Medication Schedule Templates (+Medication Calendars)
Schedule C Medications This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. They are listed on schedule c to the food and drugs. Schedule c drugs are radiopharmaceuticals, kits, and generators. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Drugs, substances, and certain chemicals used to make drugs are classified into five. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Schedule c drugs are radiopharmaceuticals, kits, and generators. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for. Schedule C Medications.
From www.ausmed.co.uk
Drug Scheduling Explained Ausmed Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Drugs, substances, and certain chemicals used to make drugs are. Schedule C Medications.
From apnapharmaguru.com
Schedule C and C1 Drugs and List Apna Pharma Guru Schedule C Medications This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. They are listed on schedule c to the food and drugs. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii,. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each. Schedule C Medications.
From theusualstuff.com
Schedule C Form 1040 How to Complete it? The Usual Stuff Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. They are listed on schedule c to the food and drugs. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule,. Schedule C Medications.
From www.youtube.com
Schedule C, C1, H, H1, K, P, M, N and X CH3 L3 Drugs and Schedule C Medications The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Additional guidance for the manufacture and control of bulk intermediates. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. They are listed on schedule c to the food and drugs. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in.. Schedule C Medications.
From www.pinterest.com
Nursing Pharmacology Controlled Substance Act Chart Pharmacology Schedule C Medications Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule c drugs are radiopharmaceuticals, kits, and generators. This regulatory roadmap gives comprehensive, general information about. Schedule C Medications.
From www.youtube.com
Schedule C, C1, H, H1, K, P, M, N and X CH3 Part3 Drugs and Schedule C Medications This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Schedule c drugs are radiopharmaceuticals, kits, and generators. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. This regulatory roadmap gives comprehensive, general information. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule. Schedule C Medications.
From www.canada.ca
Guidance Document Drug Identification Numbers for Schedule C Drugs Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. They are listed on schedule c to the food and drugs. Schedule c drugs are radiopharmaceuticals, kits, and generators. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Schedule i drugs require a prescription for sale. Schedule C Medications.
From ar.inspiredpencil.com
Drug Class Schedules Schedule C Medications Schedule c drugs are radiopharmaceuticals, kits, and generators. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. They are. Schedule C Medications.
From www.venngage.co
Medication Schedule Template Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule i drugs require a prescription for sale and are provided to. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. The nds program, which consists of three schedules and four categories. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications They are listed on schedule c to the food and drugs. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. This. Schedule C Medications.
From wolflawcolorado.com
Federal Drug Schedule Classification Pyramid Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of. Schedule C Medications.
From medshadow.org
Drug Classifications Schedule I, II, III, IV, V MedShadow Foundation Schedule C Medications Schedule c drugs are radiopharmaceuticals, kits, and generators. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Drugs, substances, and certain chemicals used. Schedule C Medications.
From www.inspiremalibu.com
A Brief Guide to Drug Scheduling in the United States Schedule C Medications The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Search napra’s national drug schedules (nds) database by drug name,. Schedule C Medications.
From mavink.com
Printable Fda Drug Schedule Chart Schedule C Medications The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications They are listed on schedule c to the food and drugs. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Schedule c drugs are radiopharmaceuticals, kits, and generators. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. This regulatory roadmap. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. They are listed on schedule c to the food and drugs. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct. Schedule C Medications.
From www.youtube.com
SCHEDULE C, C1 & X I PHARMACEUTICAL JURISPRUDENCE LECTURE I PHARMACIST Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Schedule c drugs are radiopharmaceuticals,. Schedule C Medications.
From www.getinflow.io
Why is my ADHD medication a 'schedule 2' controlled substance? Schedule C Medications Schedule c drugs are radiopharmaceuticals, kits, and generators. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. This regulatory roadmap gives. Schedule C Medications.
From www.printablee.com
Medication Schedule 10 Free PDF Printables Printablee Schedule C Medications The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. They are listed on schedule c to the food and drugs. Schedule i drugs require. Schedule C Medications.
From www.todaysrdh.com
Medications General Principles for Dental Hygienists Today's RDH Schedule C Medications Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Schedule c drugs are radiopharmaceuticals, kits, and generators. They are listed on schedule c to the food and drugs. This regulatory roadmap gives comprehensive, general information about. Schedule C Medications.
From solutionpharmacy.in
Drugs and Cosmetics Act,1940 and Its Rules, 1945 Solution Parmacy Schedule C Medications Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Schedule c drugs are radiopharmaceuticals, kits, and generators. They are listed on schedule c to the food and drugs. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Schedule i drugs require a prescription for sale and are provided to. Schedule C Medications.
From drugtestsinbulk.com
When and How To Test for Drugs A Guide Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Search napra’s national drug schedules (nds) database. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. They are listed on schedule c to the food and drugs. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds. Schedule C Medications.
From mungfali.com
Narcotic Drug Schedule Chart Schedule C Medications This regulatory roadmap gives comprehensive, general information about the regulation of radiopharmaceutical drugs for human use in. Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Additional guidance for the manufacture and control of bulk intermediates and. Schedule C Medications.
From overlandiop.com
Classification of Drugs Understanding Drug Schedule 15 Schedule C Medications Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale. Schedule C Medications.
From bceweb.org
Drug Schedules Chart A Visual Reference of Charts Chart Master Schedule C Medications Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules. Additional guidance for the manufacture and control of bulk intermediates and finished schedule c drugs. Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. Schedule i drugs require a prescription for sale and are provided to the. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Search napra’s national drug schedules (nds) database by drug name, keyword, schedule, and more. They are listed on schedule c to the food and drugs. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. This regulatory roadmap gives comprehensive,. Schedule C Medications.
From templatelab.com
40 Great Medication Schedule Templates (+Medication Calendars) Schedule C Medications Schedule c drugs are radiopharmaceuticals, kits, and generators. Schedule i drugs require a prescription for sale and are provided to the public by a pharmacist following the diagnosis. The nds program, which consists of three schedules and four categories of drugs (schedule i, schedule ii, schedule iii and unscheduled), outlines the conditions of sale for each drug schedule. Drugs, substances,. Schedule C Medications.