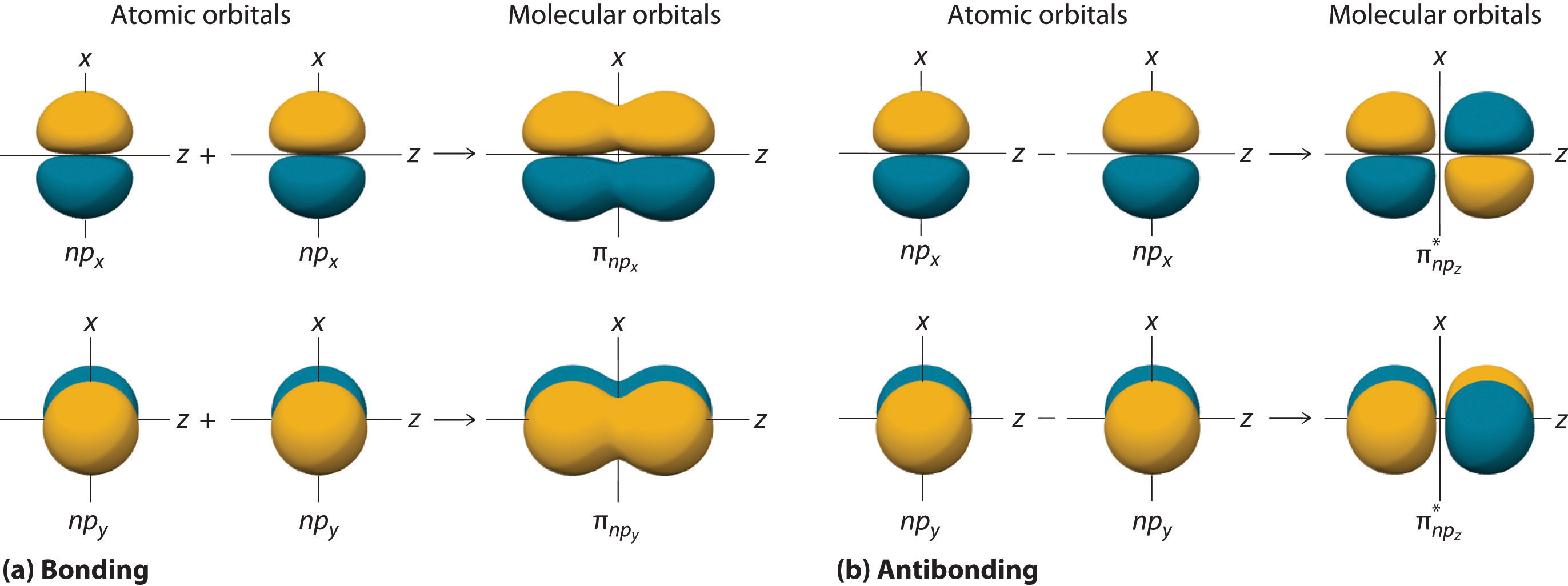
Bonding Orbital Example . bond order is the amount of bonds formed between two atoms. When atomic orbitals come into proximity and overlap, the overlap in. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. 2 if the bond order is greater then zero, a stable. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. For example, two bonds are formed between oxygen atoms, so the.
from chem.libretexts.org
When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. 2 if the bond order is greater then zero, a stable. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. bond order is the amount of bonds formed between two atoms.
Chapter 6.5 Delocalized Bonding and Molecular Orbitals Chemistry
Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. 2 if the bond order is greater then zero, a stable. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. bond order is the amount of bonds formed between two atoms. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and.
From projectopenletter.com
What Orbitals Are Used To Form The Bond Indicated In The Following Bonding Orbital Example in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. When atomic orbitals come into proximity and overlap, the overlap in. bond order is the amount of bonds formed between two atoms. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. this session on. Bonding Orbital Example.
From enginelistmoller.z13.web.core.windows.net
As Orbital Diagram Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. bond order is the amount of bonds formed between two atoms. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. 2 if the bond order is greater then zero, a stable. For example, two bonds. Bonding Orbital Example.
From chem.libretexts.org
1.7 Atomic Orbitals and Covalent Bonding Chemistry LibreTexts Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. bond order is the amount of bonds formed between two atoms. there are six degenerate. Bonding Orbital Example.
From www.researchgate.net
The GVB(SO) bonding orbitals in the 3 Σ − linear configuration of Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. bond order is the amount of bonds formed between two atoms. there are six. Bonding Orbital Example.
From socratic.org
Orbital Hybridization "Cheat Sheet"? + Example Bonding Orbital Example in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. bond order is the amount of bonds formed between two atoms. When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each. Bonding Orbital Example.
From www.logiota.com
Molecular Orbital Theory (MOT) Chemical Bonding Physical Chemistry Bonding Orbital Example there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. For example, two bonds are formed between oxygen atoms, so the. bond order is the amount of bonds formed between two atoms. When atomic orbitals come into proximity and overlap, the overlap in. this session on. Bonding Orbital Example.
From www.chemtube3d.com
Bonding orbitals in Methane sp3 hybrids Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. For example, two bonds are formed between. Bonding Orbital Example.
From chem.libretexts.org
1.7 Valence Bond Theory Chemistry LibreTexts Bonding Orbital Example there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. 2 if the bond order is greater then zero, a stable. bond order is the amount of bonds formed between two atoms. in the. Bonding Orbital Example.
From brilliant.org
chemical bonding molecular orbital theory Brilliant Math & Science Wiki Bonding Orbital Example in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. bond order is the amount of bonds formed between two atoms. 2 if the bond order. Bonding Orbital Example.
From www.vedantu.com
Orbital Overlap Definition, Detailed Explanation, Properties and FAQs Bonding Orbital Example For example, two bonds are formed between oxygen atoms, so the. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. 2 if the bond order is. Bonding Orbital Example.
From www.abpischools.org.uk
Molecular orbitals Double bonds, Ethene Bonding Orbital Example bond order is the amount of bonds formed between two atoms. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. there are six degenerate p. Bonding Orbital Example.
From saylordotorg.github.io
Localized Bonding and Hybrid Atomic Orbitals Bonding Orbital Example bond order is the amount of bonds formed between two atoms. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. 2 if the bond order is greater then zero, a stable. there are six degenerate p atomic orbitals (three from each atom) that combine to form. Bonding Orbital Example.
From mydiagram.online
[DIAGRAM] Single Phase Bonding Diagram Bonding Orbital Example in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. bond order is the amount of bonds formed between two atoms. For example, two bonds are formed between oxygen atoms, so the. this session on molecular orbital theory explains why some atoms readily form bonds with each. Bonding Orbital Example.
From madoverchemistry.wordpress.com
62.CHEMICAL BONDING (9) Covalent Bonding(8) sp3 Hybridization Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. bond order is the amount of bonds formed between two atoms. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. in the h. Bonding Orbital Example.
From chem.libretexts.org
9.5 Bonding and Antibonding Orbitals Chemistry LibreTexts Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. bond order is the. Bonding Orbital Example.
From users.highland.edu
Localized Bonding and Hybrid Atomic Orbitals Bonding Orbital Example there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. bond order is the amount of bonds formed between two atoms. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. 2 if the bond order. Bonding Orbital Example.
From www.fluxsci.com
8 Drawing Molecular Orbital Diagrams — Flux Science Bonding Orbital Example this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. When atomic orbitals come into proximity and overlap, the overlap in. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. For example, two bonds are formed between oxygen atoms, so the. there are six degenerate. Bonding Orbital Example.
From www.youtube.com
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp, Sp2, Sp3 Bonding Orbital Example Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. 2 if the bond order is greater then zero, a stable. bond order is the amount of bonds formed between two atoms. For example, two bonds are formed between oxygen atoms, so the. When atomic orbitals come into proximity and overlap, the overlap in. there are. Bonding Orbital Example.
From www.pinterest.co.uk
Pi Bond definition A bond formed when parallel orbitals overlap and Bonding Orbital Example For example, two bonds are formed between oxygen atoms, so the. 2 if the bond order is greater then zero, a stable. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. When atomic orbitals come into. Bonding Orbital Example.
From chem.libretexts.org
3.7A Orbital Overlap Chemistry LibreTexts Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. For example, two bonds are formed between oxygen atoms, so the. 2 if the bond order is greater then zero, a stable. in the h 2 molecule,. Bonding Orbital Example.
From byjus.com
A π bond is formed by the overlap of Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine. Bonding Orbital Example.
From chem.libretexts.org
2.1 Valence Bond Theory Chemistry LibreTexts Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. bond order is the amount of bonds formed between two atoms. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. this session on molecular orbital theory explains why some atoms readily form bonds with each. Bonding Orbital Example.
From brilliant.org
chemical bonding molecular orbital theory Brilliant Math & Science Wiki Bonding Orbital Example this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. bond order is the. Bonding Orbital Example.
From classnotes.org.in
Types of Molecular Orbital Formed Chemical Bonding and Molecular Bonding Orbital Example there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. When atomic orbitals come into proximity and overlap, the overlap in. bond order is the amount of bonds formed between two atoms. this session on molecular orbital theory explains why some atoms readily form bonds with. Bonding Orbital Example.
From www.chemtube3d.com
Bonding orbitals in Formaldehyde (Methanal) Bonding Orbital Example Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. For example, two bonds are formed between oxygen atoms, so the. When atomic orbitals come into proximity and overlap, the overlap in. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. 2 if the bond. Bonding Orbital Example.
From socratic.com
s,p,d,f Orbitals Chemistry Socratic Bonding Orbital Example 2 if the bond order is greater then zero, a stable. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. When atomic orbitals come into proximity and overlap, the overlap in. there are six degenerate p atomic orbitals (three from each atom) that combine to form six. Bonding Orbital Example.
From www.slideserve.com
PPT Molecular Orbital Theory PowerPoint Presentation, free download Bonding Orbital Example When atomic orbitals come into proximity and overlap, the overlap in. For example, two bonds are formed between oxygen atoms, so the. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. there are six degenerate p atomic orbitals (three from each atom) that combine to form six. Bonding Orbital Example.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry sp2 orbital Bonding Orbital Example For example, two bonds are formed between oxygen atoms, so the. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. 2 if the bond order is greater then zero, a stable. When atomic orbitals come into proximity and overlap, the overlap in. Constructive and destructive interaction of \ce. Bonding Orbital Example.
From mavink.com
Triple Bond Orbital Diagram Bonding Orbital Example For example, two bonds are formed between oxygen atoms, so the. in the h 2 molecule, for example, two singly occupied 1 s atomic orbitals combine to form two molecular orbitals. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. there are six degenerate p atomic. Bonding Orbital Example.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry sp2 orbital Bonding Orbital Example bond order is the amount of bonds formed between two atoms. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. When atomic orbitals come into. Bonding Orbital Example.
From www.coursehero.com
[Solved] sketch sigma and pi bond from p orbital Course Hero Bonding Orbital Example this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. 2 if the bond order is greater then zero, a stable. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. bond order is the amount of bonds formed between two atoms. in the h. Bonding Orbital Example.
From chem.libretexts.org
Chapter 6.5 Delocalized Bonding and Molecular Orbitals Chemistry Bonding Orbital Example Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. bond order is the amount of bonds formed between two atoms. 2 if the bond order is greater then zero, a stable. there are six. Bonding Orbital Example.
From www.chemtube3d.com
Bonding orbitals in Ethylene (Ethene) sp2 Bonding Orbital Example For example, two bonds are formed between oxygen atoms, so the. bond order is the amount of bonds formed between two atoms. there are six degenerate p atomic orbitals (three from each atom) that combine to form six molecular orbitals, three bonding and. Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. in the. Bonding Orbital Example.
From saylordotorg.github.io
Localized Bonding and Hybrid Atomic Orbitals Bonding Orbital Example Constructive and destructive interaction of \ce {h_ {2}} hx 2 molecular orbitals. 2 if the bond order is greater then zero, a stable. When atomic orbitals come into proximity and overlap, the overlap in. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. For example, two bonds are. Bonding Orbital Example.
From chem.libretexts.org
Chapter 6.6 Polyatomic Systems, Multiple Bonds, Resonance Chemistry Bonding Orbital Example 2 if the bond order is greater then zero, a stable. For example, two bonds are formed between oxygen atoms, so the. this session on molecular orbital theory explains why some atoms readily form bonds with each other and other atoms don’t. there are six degenerate p atomic orbitals (three from each atom) that combine to form six. Bonding Orbital Example.