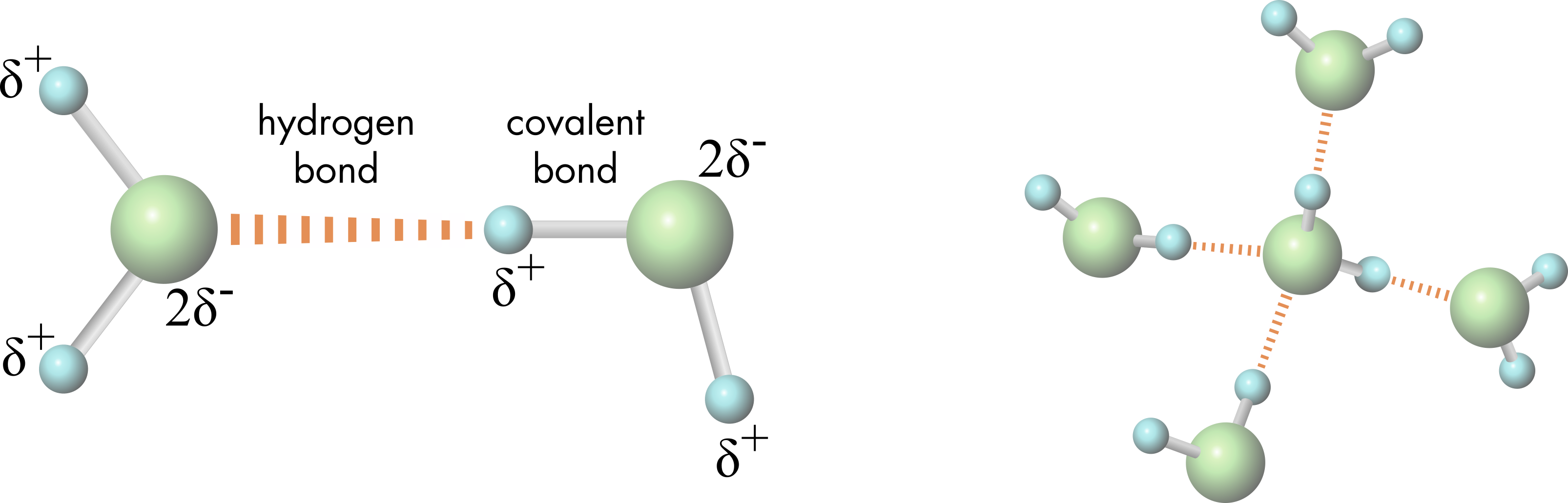Is Table Salt A Hydrogen Bond . for example, two hydrogen atoms bond covalently to form an h 2 molecule; the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Each hydrogen atom in the h 2 molecule shares. Two requirements for hydrogen bonding: First molecules has hydrogen attached to a highly electronegative. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Instead, it contains many ions. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions having an. there are two requirements for hydrogen bonding. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded.
from ar.inspiredpencil.com
table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). the molecular formula of table salt—sodium chloride—is nacl. Two requirements for hydrogen bonding: First molecules has hydrogen attached to a highly electronegative. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. Each hydrogen atom in the h 2 molecule shares. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Instead, it contains many ions. for example, two hydrogen atoms bond covalently to form an h 2 molecule;
Water Molecule Hydrogen Bond Diagram
Is Table Salt A Hydrogen Bond there are two requirements for hydrogen bonding. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Two requirements for hydrogen bonding: there are two requirements for hydrogen bonding. for example, two hydrogen atoms bond covalently to form an h 2 molecule; table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). the molecular formula of table salt—sodium chloride—is nacl. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Each hydrogen atom in the h 2 molecule shares. In the solid lattice, each ion is surrounded by six ions having an. First molecules has hydrogen attached to a highly electronegative. Instead, it contains many ions. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded.
From www.vrogue.co
Formation Of Hydrogen Bonds In Water Easybiologyclass vrogue.co Is Table Salt A Hydrogen Bond Two requirements for hydrogen bonding: In the solid lattice, each ion is surrounded by six ions having an. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). First molecules has hydrogen attached to a highly electronegative. there are two requirements for hydrogen bonding. the molecular formula of table salt—sodium. Is Table Salt A Hydrogen Bond.
From www.expii.com
Hydrogen Bonding — Definition & Overview Expii Is Table Salt A Hydrogen Bond there are two requirements for hydrogen bonding. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. Instead, it contains many ions. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Each hydrogen atom in the h 2 molecule shares.. Is Table Salt A Hydrogen Bond.
From thelifepile.com
Know Your Salt The Difference Between Table Salt, Kosher Salt & Sea Salt Is Table Salt A Hydrogen Bond for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. for example, two hydrogen atoms bond covalently to form an h 2 molecule; Each hydrogen atom in the. Is Table Salt A Hydrogen Bond.
From animalia-life.club
Hydrogen Bond Is Table Salt A Hydrogen Bond In the solid lattice, each ion is surrounded by six ions having an. Two requirements for hydrogen bonding: the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. the molecular formula of table salt—sodium chloride—is nacl. there are two requirements for hydrogen bonding. First molecules has hydrogen attached. Is Table Salt A Hydrogen Bond.
From cabinet.matttroy.net
Is Table Salt A Molecule Or Compound Matttroy Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Two requirements for hydrogen bonding: for example, two hydrogen atoms bond covalently to form an h 2 molecule; for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded.. Is Table Salt A Hydrogen Bond.
From www.huffingtonpost.com
Of Salt, Saltation and Salience The Case for Fixing What's Broken Is Table Salt A Hydrogen Bond In the solid lattice, each ion is surrounded by six ions having an. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Instead, it contains many ions. for example, h a. Is Table Salt A Hydrogen Bond.
From www.hotzxgirl.com
Cohsive Hydrogen Bond Evaporation Water Molecule Hot Sex Picture Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions having an. Instead, it contains many ions. for example, h a 2 o is a covalent compound because it contains hydrogen and. Is Table Salt A Hydrogen Bond.
From studiousguy.com
9 Hydrogen Bond Examples in Real Life StudiousGuy Is Table Salt A Hydrogen Bond Two requirements for hydrogen bonding: the molecular formula of table salt—sodium chloride—is nacl. Each hydrogen atom in the h 2 molecule shares. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). In the solid lattice, each ion is surrounded by six ions having an. First molecules has hydrogen attached to. Is Table Salt A Hydrogen Bond.
From chemistryskills.com
Hydrogen Bonding Chemistry Skills Is Table Salt A Hydrogen Bond In the solid lattice, each ion is surrounded by six ions having an. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Each hydrogen atom in the h 2 molecule shares.. Is Table Salt A Hydrogen Bond.
From www.okenergytoday.com
World’s largest hydrogen storage site planned in South Texas Oklahoma Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; First molecules has hydrogen attached to a highly electronegative. Two requirements for hydrogen bonding: the molecular formula of table salt—sodium chloride—is nacl. there are two requirements for hydrogen bonding. In the solid lattice, each ion is surrounded by six ions having. Is Table Salt A Hydrogen Bond.
From www.youtube.com
007Hydrogen Bonding & Water YouTube Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; In the solid lattice, each ion is surrounded by six ions having an. Two requirements for hydrogen bonding: table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). Each hydrogen atom in the h 2. Is Table Salt A Hydrogen Bond.
From www.science-revision.co.uk
Hydrogen bonding Is Table Salt A Hydrogen Bond Instead, it contains many ions. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). In the solid lattice, each ion is surrounded by six ions having an. the molecular formula of table salt—sodium chloride—is nacl. there are two requirements for hydrogen bonding. Each hydrogen atom in the h 2. Is Table Salt A Hydrogen Bond.
From www.differencebetween.com
Difference Between Salt Bridge and Hydrogen Bond Compare the Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; First molecules has hydrogen attached to a highly electronegative. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). In the solid lattice, each ion is surrounded by six ions having an. Instead, it contains. Is Table Salt A Hydrogen Bond.
From www.researchgate.net
Scheme of hydrogen bonds in sodium carboxymethylcellulose aqueous Is Table Salt A Hydrogen Bond there are two requirements for hydrogen bonding. First molecules has hydrogen attached to a highly electronegative. Instead, it contains many ions. for example, two hydrogen atoms bond covalently to form an h 2 molecule; the molecular formula of table salt—sodium chloride—is nacl. for example, h a 2 o is a covalent compound because it contains hydrogen. Is Table Salt A Hydrogen Bond.
From driesvannotendesigner.blogspot.com
learning through art water molecules and hydrogen bonding Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. First molecules has hydrogen attached to a highly electronegative. Each hydrogen atom in the h 2 molecule shares. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. . Is Table Salt A Hydrogen Bond.
From brokeasshome.com
Table Salt Vs Kosher For Brining Meat Dopamine Levels In The Brain Is Table Salt A Hydrogen Bond In the solid lattice, each ion is surrounded by six ions having an. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). Each hydrogen atom in the h 2 molecule. Is Table Salt A Hydrogen Bond.
From www.thesciencehive.co.uk
Alcohols* — the science sauce Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Each hydrogen atom in the h 2 molecule shares. First molecules has hydrogen attached to a highly electronegative. the molecular formula of table salt—sodium chloride—is nacl. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water. Is Table Salt A Hydrogen Bond.
From isaacscienceblog.com
Hydrogen bonding Isaac's science blog Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. First molecules has hydrogen attached to a highly electronegative. for example, h a 2 o is a covalent compound because it contains. Is Table Salt A Hydrogen Bond.
From www.echemi.com
How can the hydrogen bonds in solid HF be best represented? ECHEMI Is Table Salt A Hydrogen Bond the molecular formula of table salt—sodium chloride—is nacl. Instead, it contains many ions. Two requirements for hydrogen bonding: In the solid lattice, each ion is surrounded by six ions having an. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. for example, two hydrogen atoms bond covalently. Is Table Salt A Hydrogen Bond.
From www.researchgate.net
Hydrogenbond donor/ acceptor pattern exposed in the major groove. (A Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. the molecular formula of table salt—sodium chloride—is nacl. Each hydrogen atom in the h 2 molecule shares. In the solid lattice, each ion is surrounded by six ions having an. for example, two hydrogen atoms bond covalently to. Is Table Salt A Hydrogen Bond.
From www.wizeprep.com
Intermolecular Forces Hydrogen Bond Wize University Biology Textbook Is Table Salt A Hydrogen Bond Instead, it contains many ions. there are two requirements for hydrogen bonding. In the solid lattice, each ion is surrounded by six ions having an. Two requirements for hydrogen bonding: Each hydrogen atom in the h 2 molecule shares. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently. Is Table Salt A Hydrogen Bond.
From www.nagwa.com
Question Video Identifying the Bond Enthalpies of Hydrogen Bonds Nagwa Is Table Salt A Hydrogen Bond there are two requirements for hydrogen bonding. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Instead, it contains many ions. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; First molecules has hydrogen attached to a highly electronegative. . Is Table Salt A Hydrogen Bond.
From www.chegg.com
Solved Reterences A molecule or ion that donates the Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Each hydrogen atom in the h 2 molecule shares. In the solid lattice, each ion is surrounded by six ions having an. Two requirements for hydrogen bonding: the molecular formula of table salt—sodium chloride—is nacl. table salt, or sodium chloride (\(\mathrm{nacl}\)),. Is Table Salt A Hydrogen Bond.
From biosynthesisthomas.blogspot.com
BioSynthesis Chapter 2 The Chemical Basis of Life I Atoms, Molecules Is Table Salt A Hydrogen Bond the molecular formula of table salt—sodium chloride—is nacl. Each hydrogen atom in the h 2 molecule shares. Instead, it contains many ions. First molecules has hydrogen attached to a highly electronegative. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. table salt, or sodium chloride (\(\mathrm{nacl}\)), the. Is Table Salt A Hydrogen Bond.
From www.wisegeek.net
What Are the Different Uses for Onion Salt? (with pictures) Is Table Salt A Hydrogen Bond Each hydrogen atom in the h 2 molecule shares. for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. Instead, it contains many ions. for example, two hydrogen atoms bond covalently to form an h 2 molecule; the bonds in salt compounds are called ionic because they. Is Table Salt A Hydrogen Bond.
From sciencenotes.org
Types of Chemical Bonds Is Table Salt A Hydrogen Bond First molecules has hydrogen attached to a highly electronegative. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). there are two requirements for hydrogen bonding. Each hydrogen atom in the h 2 molecule shares. for example, h a 2 o is a covalent compound because it contains hydrogen and. Is Table Salt A Hydrogen Bond.
From mydiagram.online
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding Is Table Salt A Hydrogen Bond Two requirements for hydrogen bonding: table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively.. Is Table Salt A Hydrogen Bond.
From www.pinterest.com
Sea Salt vs. Table Salt Table salt, Nutritional therapy association Is Table Salt A Hydrogen Bond In the solid lattice, each ion is surrounded by six ions having an. the molecular formula of table salt—sodium chloride—is nacl. First molecules has hydrogen attached to a highly electronegative. Each hydrogen atom in the h 2 molecule shares. Instead, it contains many ions. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in. Is Table Salt A Hydrogen Bond.
From millgulf.weebly.com
Periodic table hydrogen atomic number millgulf Is Table Salt A Hydrogen Bond for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. there are two requirements for hydrogen bonding. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). Instead, it contains many ions. the molecular formula of table salt—sodium chloride—is. Is Table Salt A Hydrogen Bond.
From openpress.usask.ca
5.2 Bonding and Lattices Physical Geology, First University of Is Table Salt A Hydrogen Bond table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Instead, it contains many ions. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Each hydrogen atom in the h 2 molecule shares. In the solid lattice, each ion is surrounded by. Is Table Salt A Hydrogen Bond.
From ar.inspiredpencil.com
Water Molecule Hydrogen Bond Diagram Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; Each hydrogen atom in the h 2 molecule shares. First molecules has hydrogen attached to a highly electronegative. for example, h a. Is Table Salt A Hydrogen Bond.
From mungfali.com
Hydrogen Bonding Forces Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. for example, two hydrogen atoms bond covalently to form an h 2 molecule; for example, h a 2 o is a covalent compound because it contains hydrogen and oxygen (different elements) covalently bonded. there are two requirements. Is Table Salt A Hydrogen Bond.
From whatsinsight.org
Sodium Hydrogen Sulfate What's Insight Is Table Salt A Hydrogen Bond table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). the molecular formula of table salt—sodium chloride—is nacl. the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. Instead, it contains many ions. for example, h a 2 o is. Is Table Salt A Hydrogen Bond.
From physicstuff.com
What is anomalous expansion of water? PhysicStuff Is Table Salt A Hydrogen Bond there are two requirements for hydrogen bonding. table salt, or sodium chloride (\(\mathrm{nacl}\)), the most common ionic compound, is soluble in water (\(360 \mathrm{~g/l}\)). table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; First molecules has hydrogen attached to a highly electronegative. the bonds in salt compounds are called. Is Table Salt A Hydrogen Bond.
From mungfali.com
Hydrogen Bonding Forces Is Table Salt A Hydrogen Bond the bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively. there are two requirements for hydrogen bonding. for example, two hydrogen atoms bond covalently to form an h 2 molecule; table salt, like many ionic compounds, doesn't consist of just one sodium and one chloride ion; . Is Table Salt A Hydrogen Bond.