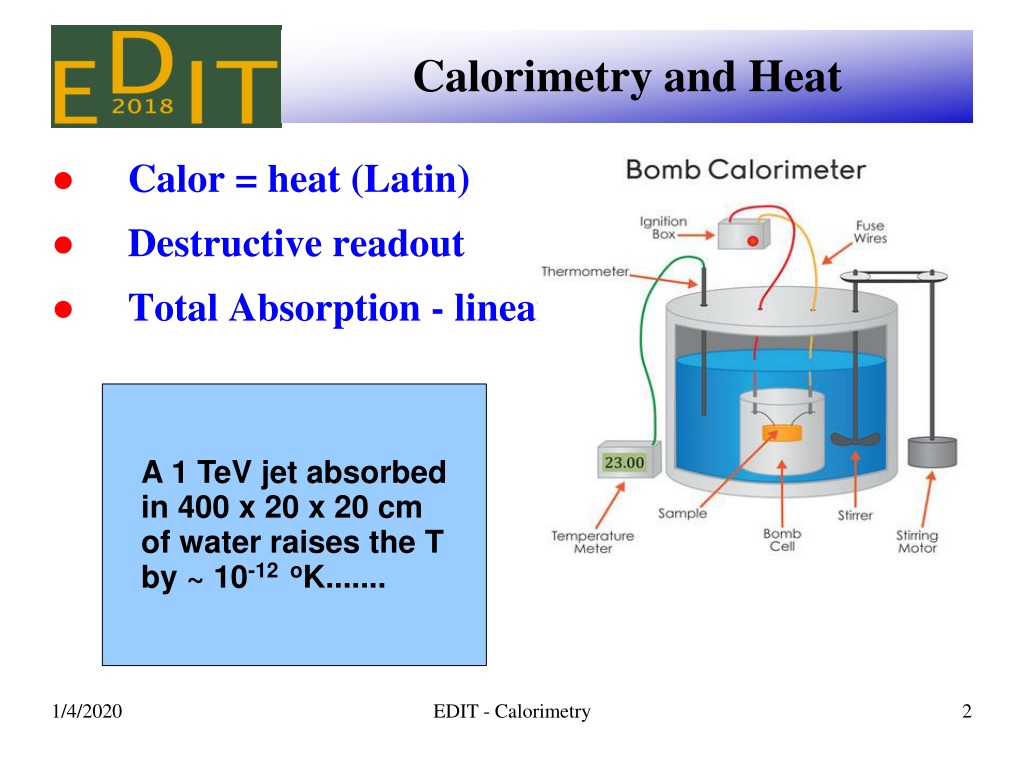
Why Is Calorimetry So Important . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. In order to measure the heat. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics.
from www.slideserve.com
Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. In order to measure the heat. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic.
PPT Calorimetry PowerPoint Presentation, free download ID9539914
Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. In order to measure the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5577734 Why Is Calorimetry So Important Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Why Is Calorimetry So Important For example, when an exothermic. Chemists use calorimetry to determine the. In order to measure the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Because calorimetry is used to measure the heat of. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Why Is Calorimetry So Important Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Chemists use calorimetry to determine the. In order to measure the heat.. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1609320 Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. In order to measure the heat. Chemists use calorimetry to determine the. Because calorimetry is used to measure the heat of a reaction, it is a crucial. Why Is Calorimetry So Important.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Why Is Calorimetry So Important Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Why Is Calorimetry So Important.
From chem.libretexts.org
5.3 Calorimetry Chemistry LibreTexts Why Is Calorimetry So Important Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. In order to measure the heat. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Why Is Calorimetry So Important.
From users.highland.edu
Calorimetry Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure amounts of heat transferred to. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. For example, when an exothermic. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. Chemists use calorimetry. Why Is Calorimetry So Important.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Why Is Calorimetry So Important To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. In order to measure the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Why Is Calorimetry So Important In order to measure the heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Chemists use calorimetry to determine the. For example, when an. Why Is Calorimetry So Important.
From www.youtube.com
Principle of Calorimetry YouTube Why Is Calorimetry So Important Chemists use calorimetry to determine the. In order to measure the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Why Is Calorimetry So Important Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the. Why Is Calorimetry So Important.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Why Is Calorimetry So Important To do so, the heat is exchanged with a calibrated object (calorimeter). Chemists use calorimetry to determine the. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure. Why Is Calorimetry So Important.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Because calorimetry is used to. Why Is Calorimetry So Important.
From studyfinder.org
How to Use a Calorimetry Gizmo to Get the Answers You Need Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. Calorimetry is used to. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3207019 Why Is Calorimetry So Important Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or. Why Is Calorimetry So Important.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Why Is Calorimetry So Important To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. In order to measure the heat. Because calorimetry is used. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Chapter 16 Calorimetry PowerPoint Presentation, free download Why Is Calorimetry So Important For example, when an exothermic. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. Calorimetry is a crucial technique. Why Is Calorimetry So Important.
From studylib.net
Calorimetry Why Is Calorimetry So Important To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. For example, when an exothermic reaction occurs in solution in a calorimeter, the. For example, when an exothermic. In order to measure the. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Why Is Calorimetry So Important In order to measure the heat. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Because calorimetry is used to measure the heat. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Review of Modern Calorimetry for Complex Fluids and Biology Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. Calorimetry is used to measure the amount of thermal energy. Why Is Calorimetry So Important.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Why Is Calorimetry So Important Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. For example, when an exothermic reaction occurs in solution. Why Is Calorimetry So Important.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Why Is Calorimetry So Important A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. To do so, the heat is exchanged with a. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Why Is Calorimetry So Important For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the. Why Is Calorimetry So Important.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Why Is Calorimetry So Important Chemists use calorimetry to determine the. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. In order to measure the heat. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to. Why Is Calorimetry So Important.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Chemists use calorimetry to determine the. A. Why Is Calorimetry So Important.
From studylib.net
Calorimetry Why Is Calorimetry So Important For example, when an exothermic. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a crucial technique in thermodynamics,. Why Is Calorimetry So Important.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Why Is Calorimetry So Important To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Because calorimetry is used to measure the heat of a reaction, it. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9539914 Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Why Is Calorimetry So Important Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. To do so, the heat is. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID2765956 Why Is Calorimetry So Important Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. Why Is Calorimetry So Important.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Why Is Calorimetry So Important Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Because calorimetry is used to measure the heat of a reaction, it. Why Is Calorimetry So Important.
From iqclasses.in
calorimetry chapter important notes class10 icse Why Is Calorimetry So Important Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. Chemists use calorimetry to determine the. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. For example, when. Why Is Calorimetry So Important.
From www.youtube.com
050 Calorimetry YouTube Why Is Calorimetry So Important For example, when an exothermic. Calorimetry is a crucial technique in thermodynamics, the study of heat transfer and energy conversion. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to. Why Is Calorimetry So Important.