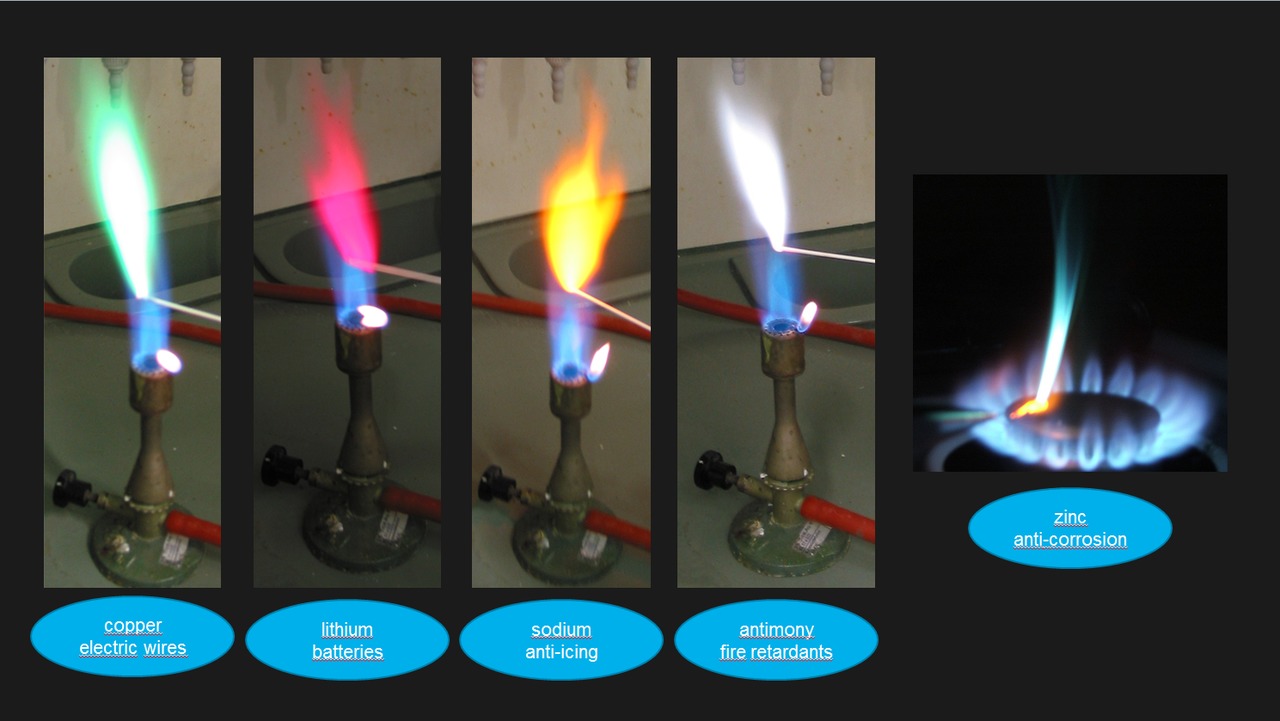
Zinc Carbonate Flame Test . to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. After doing this lab, you'll be able to explain how the flame test works and. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. in this lab, we'll be learning about flame tests. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample.
from peda.net
Flame tests are used to identify the presence of a relatively small number of metal ions in a compound other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. After doing this lab, you'll be able to explain how the flame test works and. in this lab, we'll be learning about flame tests. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an.
Flame tests
Zinc Carbonate Flame Test this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. After doing this lab, you'll be able to explain how the flame test works and. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.
From ar.inspiredpencil.com
Flame Test Calcium Carbonate Zinc Carbonate Flame Test other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how. Zinc Carbonate Flame Test.
From www.youtube.com
Thermal of Zinc Carbonate YouTube Zinc Carbonate Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. Flame tests are used to identify the presence of a relatively small number of. Zinc Carbonate Flame Test.
From www.youtube.com
Potassium carbonate flame test. YouTube Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. the. Zinc Carbonate Flame Test.
From peda.net
Flame tests Zinc Carbonate Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. After doing this lab, you'll be able to explain how the flame test works and. in this lab, we'll be learning about flame tests. other elements that can impart a blue color to a flame. Zinc Carbonate Flame Test.
From pixels.com
Strontium Metal Flame Test Photograph by Andrew Mcclenaghan/science Zinc Carbonate Flame Test this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. to perform flame tests of. Zinc Carbonate Flame Test.
From www.youtube.com
Mrs WP's flame testfor zinc YouTube Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. After doing this lab, you'll be able to explain how the flame test. Zinc Carbonate Flame Test.
From exobygfvi.blob.core.windows.net
Is A Flame Test A Chemical Change at Tim Crow blog Zinc Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. in this lab, we'll be learning about flame tests. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. other elements that can impart a. Zinc Carbonate Flame Test.
From fphoto.photoshelter.com
science chemistry flame test Fundamental Photographs The Art of Science Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. in this lab, we'll. Zinc Carbonate Flame Test.
From dxoqbloyt.blob.core.windows.net
Flame Test Lab The Identification Of An Element Answers at Linda Zinc Carbonate Flame Test other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. After doing this lab, you'll be able to explain how the flame test works and. Flame tests. Zinc Carbonate Flame Test.
From chemistry.com.pk
Metal Ion Flame Test Colours [Infographic] Zinc Carbonate Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. in this lab, we'll be learning about flame tests. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound the flame test is a qualitative test. Zinc Carbonate Flame Test.
From www.thoughtco.com
Flame Test Colors Photo Gallery Zinc Carbonate Flame Test other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. After doing this lab, you'll be able to explain how the flame test works and. . Zinc Carbonate Flame Test.
From www.thoughtco.com
Flame Test Colors Photo Gallery Zinc Carbonate Flame Test the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed. Zinc Carbonate Flame Test.
From www.semanticscholar.org
Figure 1 from Zinc Hydroxystannate or Zinc Stannatecoated Calcium Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. . Zinc Carbonate Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame. Zinc Carbonate Flame Test.
From simple.wikipedia.org
FileZinc flame test 2.jpg Simple English Wikipedia, the free Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. this. Zinc Carbonate Flame Test.
From testbook.com
Zinc Carbonate Learn its Formula, Structure, Properties & Uses Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound this page describes how to. Zinc Carbonate Flame Test.
From studylib.net
The Flame Test Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. other elements that can impart a blue color to a flame test. Zinc Carbonate Flame Test.
From ar.inspiredpencil.com
Flame Test Calcium Carbonate Zinc Carbonate Flame Test in this lab, we'll be learning about flame tests. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. this page describes how to do a. Zinc Carbonate Flame Test.
From www.youtube.com
Qualitative Flame Test BaCl Barium Chloride YouTube Zinc Carbonate Flame Test this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. After doing this. Zinc Carbonate Flame Test.
From www.writework.com
Experiment to describe reactivity of a given set of metals. WriteWork Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame. Zinc Carbonate Flame Test.
From www.thoughtco.com
How to Do a Flame Test for Qualitative Analysis Zinc Carbonate Flame Test the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in this lab, we'll be learning about flame tests. After doing this lab, you'll be able to explain how the flame test works and. Flame tests are used to identify the presence of a relatively small number of metal. Zinc Carbonate Flame Test.
From ar.inspiredpencil.com
Flame Test Colors Zinc Zinc Carbonate Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. After doing this lab, you'll be able to explain how the flame test works and. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests. Zinc Carbonate Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound other. Zinc Carbonate Flame Test.
From www.thoughtco.com
Flame Test Colors Photo Gallery Zinc Carbonate Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. . Zinc Carbonate Flame Test.
From www.thoughtco.com
Flame Test Colors Photo Gallery Zinc Carbonate Flame Test the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. After doing this lab, you'll be able to explain how the flame test works and. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound other elements that can impart. Zinc Carbonate Flame Test.
From www.youtube.com
FLAME TEST Na, Cu, Ca, K, Zn, Fe YouTube Zinc Carbonate Flame Test After doing this lab, you'll be able to explain how the flame test works and. this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. Flame tests are used to identify the presence of a relatively. Zinc Carbonate Flame Test.
From ofite.com
OFI Testing Equipment, Inc. Zinc Carbonate Test Kit Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in this lab, we'll be learning about flame tests. Flame tests are used to identify. Zinc Carbonate Flame Test.
From www.youtube.com
The action of heat on zinc II nitrate Laboratory experiments on salts Zinc Carbonate Flame Test The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. After doing this lab, you'll be able to explain how the flame test works and. to. Zinc Carbonate Flame Test.
From www.youtube.com
flame test of barium ,strontium , calcium,zinc, copper YouTube Zinc Carbonate Flame Test this page describes how to do a flame test for a range of metal ions, and briefly describes how the flame colour arises. in this lab, we'll be learning about flame tests. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. to perform flame tests of. Zinc Carbonate Flame Test.
From fphoto.photoshelter.com
science chemistry flame test Fundamental Photographs The Art of Science Zinc Carbonate Flame Test in this lab, we'll be learning about flame tests. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify. Zinc Carbonate Flame Test.
From www.toppr.com
(c) How would you distinguish between the following pairs of substances Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. the flame test is a qualitative test in analytical chemistry used to. Zinc Carbonate Flame Test.
From www.sciencecompany.com
Flame Test Chemical Kit With Five Chemicals from the Science Company. Zinc Carbonate Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. the flame test is a qualitative test in analytical chemistry used to. Zinc Carbonate Flame Test.
From www.youtube.com
AQA/OCR GCSE and A Level test for cation and anion ions carbonate Zinc Carbonate Flame Test other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. to perform flame tests of metal cations in order to observe their characteristic colors, to match. Zinc Carbonate Flame Test.
From www.youtube.com
Potassium carbonate flame test. YouTube Zinc Carbonate Flame Test the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors. Zinc Carbonate Flame Test.
From ditheodamme.com
Why Are Flame Tests Essential For Identifying Alkali Metals? Zinc Carbonate Flame Test other elements that can impart a blue color to a flame test are zinc, selenium, antimony, arsenic, lead, and. in this lab, we'll be learning about flame tests. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests are used to identify the presence of a. Zinc Carbonate Flame Test.