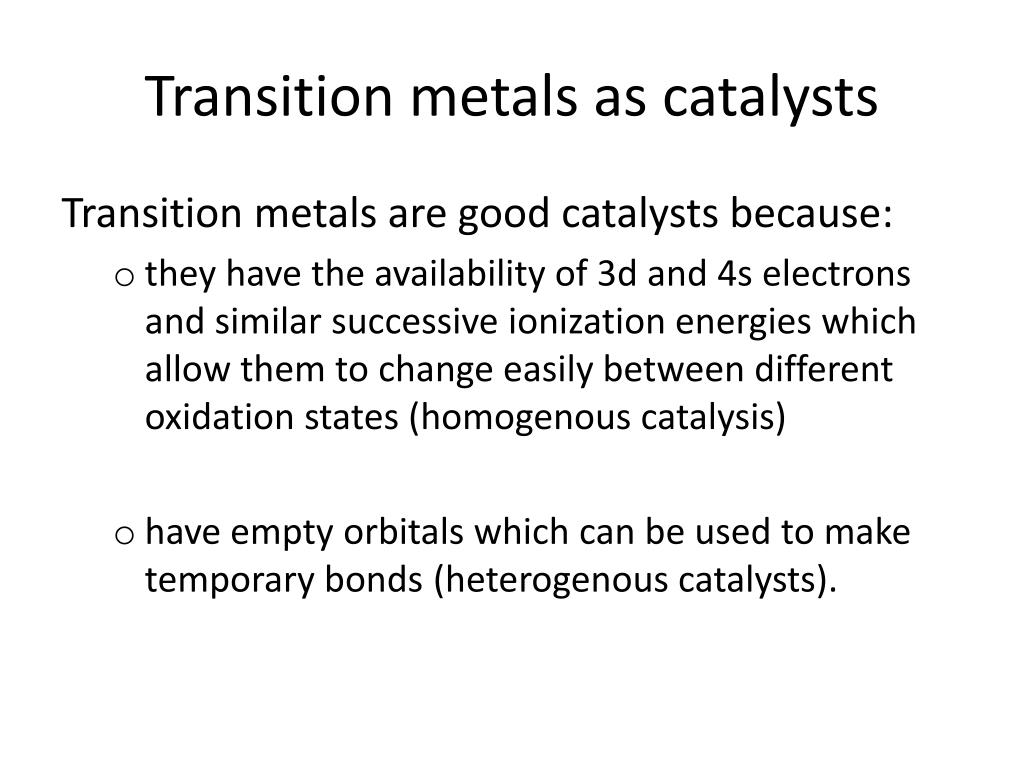
Catalysts For Transition Metals . Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to.
from www.slideserve.com
the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. the transition metals on the periodic table. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to.
PPT Transition metals catalysts PowerPoint Presentation, free
Catalysts For Transition Metals the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to.
From en.nsrl.ustc.edu.cn
Synthesis and Catalytic Performance of Transition Metal Catalysts Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the ability of transition metals to shuttle between different oxidation states. Catalysts For Transition Metals.
From chemisfast.blogspot.com
Why most of the transition metals are used as catalysts ? PG.CHEMEASY Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up. Catalysts For Transition Metals.
From www.mdpi.com
Catalysts Free FullText Influence of Transition Metal on the Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. transition metals and their compounds function. Catalysts For Transition Metals.
From www.slideserve.com
PPT Transition metals catalysts PowerPoint Presentation, free Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the transition metals on the periodic table. the ability of transition metals to. Catalysts For Transition Metals.
From www.slideserve.com
PPT Metalion catalysis Metals can function catalytically in several Catalysts For Transition Metals Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the. Catalysts For Transition Metals.
From www.youtube.com
Transition Metals as Catalysts Alevel Chemistry OCR, AQA, Edexcel Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. the transition. Catalysts For Transition Metals.
From www.mdpi.com
Catalysts Free FullText Tunable LateTransitionMetalCatalyzed Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. the transition metals on the periodic table. transition metals and their compounds function as catalysts either because. Catalysts For Transition Metals.
From www.youtube.com
A.3 Transition metal catalysts (SL) YouTube Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between.. Catalysts For Transition Metals.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalysts For Transition Metals these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the transition metals on the periodic table. the ability of transition metals to shuttle between. Catalysts For Transition Metals.
From studylib.net
Transition Metals & Catalysis Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals on the periodic table. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the ability of. Catalysts For Transition Metals.
From phys.org
Teaching old transition metals new tricks Chemists activate palladium Catalysts For Transition Metals the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. transition metals and their compounds function as catalysts either because of their ability to change oxidation state. Catalysts For Transition Metals.
From www.tes.com
New AQA A2 Organic chemistry Transition metals catalysts Teaching Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of. Catalysts For Transition Metals.
From www.slideserve.com
PPT Transition Metals PowerPoint Presentation, free download ID2422197 Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the transition metals on the periodic table. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use. Catalysts For Transition Metals.
From www.mdpi.com
Catalysts Free FullText Layered Ternary and Quaternary Transition Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. . Catalysts For Transition Metals.
From www.mdpi.com
Transition Metals in Catalysis MDPI Books Catalysts For Transition Metals these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. the ability of transition metals to shuttle between different. Catalysts For Transition Metals.
From www.tes.com
Transition Metals Catalysts Teaching Resources Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. this is one reason why transition metals make such excellent catalysts, they. Catalysts For Transition Metals.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to. Catalysts For Transition Metals.
From studylib.net
TRANSITION METALS AS CATALYSTS Catalysts For Transition Metals Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used. Catalysts For Transition Metals.
From www.mdpi.com
Organic Synthesis via Transition MetalCatalysis MDPI Books Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation. Catalysts For Transition Metals.
From europepmc.org
Metal Catalysts for Heterogeneous Catalysis From Single Atoms to Catalysts For Transition Metals these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. the transition metals on the periodic table. transition metals and their compounds function as catalysts either because of their ability to. Catalysts For Transition Metals.
From www.mdpi.com
Catalysts Free FullText SingleAtom Transition Metal Catalysts For Transition Metals Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. the ability. Catalysts For Transition Metals.
From mungfali.com
Ppt Chapter 20 The Transition Elements Powerpoint Presentation, Free 219 Catalysts For Transition Metals the transition metals on the periodic table. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier. Catalysts For Transition Metals.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Transition metals Heterogeneous catalysis Catalysts For Transition Metals the transition metals on the periodic table. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use of transition metals and. Catalysts For Transition Metals.
From www.youtube.com
Transition metals as catalysts YouTube Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals on the periodic table. the crucial factors. Catalysts For Transition Metals.
From www.researchgate.net
(PDF) UNIT IV CATALYSIS (Transitionmetal and Organocatalysis in Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the. Catalysts For Transition Metals.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or,. Catalysts For Transition Metals.
From www.youtube.com
DM Transtion Metals as Catalysts YouTube Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the ability of transition metals to shuttle between different oxidation states. Catalysts For Transition Metals.
From www.slideserve.com
PPT Transition metals catalysts PowerPoint Presentation, free Catalysts For Transition Metals the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to.. Catalysts For Transition Metals.
From www.science.org
A supramolecular microenvironment strategy for transition metal Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. the ability of transition metals to. Catalysts For Transition Metals.
From www.science.org
Using nature’s blueprint to expand catalysis with Earthabundant metals Catalysts For Transition Metals this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals on the periodic table. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. transition metals and their compounds function as catalysts either because of their ability to change. Catalysts For Transition Metals.
From www.slideshare.net
TRANSITION METAL CATALYSIS Catalysts For Transition Metals the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. the transition metals. Catalysts For Transition Metals.
From www.researchgate.net
(PDF) Transition Metal Catalysis in Confined Spaces Catalysts For Transition Metals transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. this is one reason why transition metals make such excellent catalysts, they are able to make. Catalysts For Transition Metals.
From pubs.rsc.org
Transition metalbased catalysts for the electrochemical CO 2 reduction Catalysts For Transition Metals the transition metals on the periodic table. these observations led to the use of transition metals and their compounds to facilitate chemistry experiments. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. this is one reason why transition metals make such excellent catalysts, they are able to. Catalysts For Transition Metals.
From www.cell.com
Enantioselective CH Bond Functionalizations by 3d TransitionMetal Catalysts For Transition Metals the transition metals on the periodic table. the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. the crucial factors that are used to tune the activity of the catalyst towards oer are summarized, including. these observations led to the use of transition metals and their compounds. Catalysts For Transition Metals.
From pubs.rsc.org
Transition metal catalysis in confined spaces Chemical Society Catalysts For Transition Metals the ability of transition metals to shuttle between different oxidation states while forming complexes with the reagents in a. Catalysts are compounds that speed up chemical reactions by lowering the energy barrier between. this is one reason why transition metals make such excellent catalysts, they are able to make and break bonds to ligands. the transition metals. Catalysts For Transition Metals.