Fda Labeling Requirements For Medications . This paragraph applies only to prescription drug. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. (d) labeling requirements for new and more recently approved prescription drug products. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. (a) general requirements. Prescription drug labeling described in § 201.100(d) must meet the following. search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site.
from www.slideserve.com
The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug products. Prescription drug labeling described in § 201.100(d) must meet the following. (a) general requirements. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. This paragraph applies only to prescription drug. search for labels on dailymed.
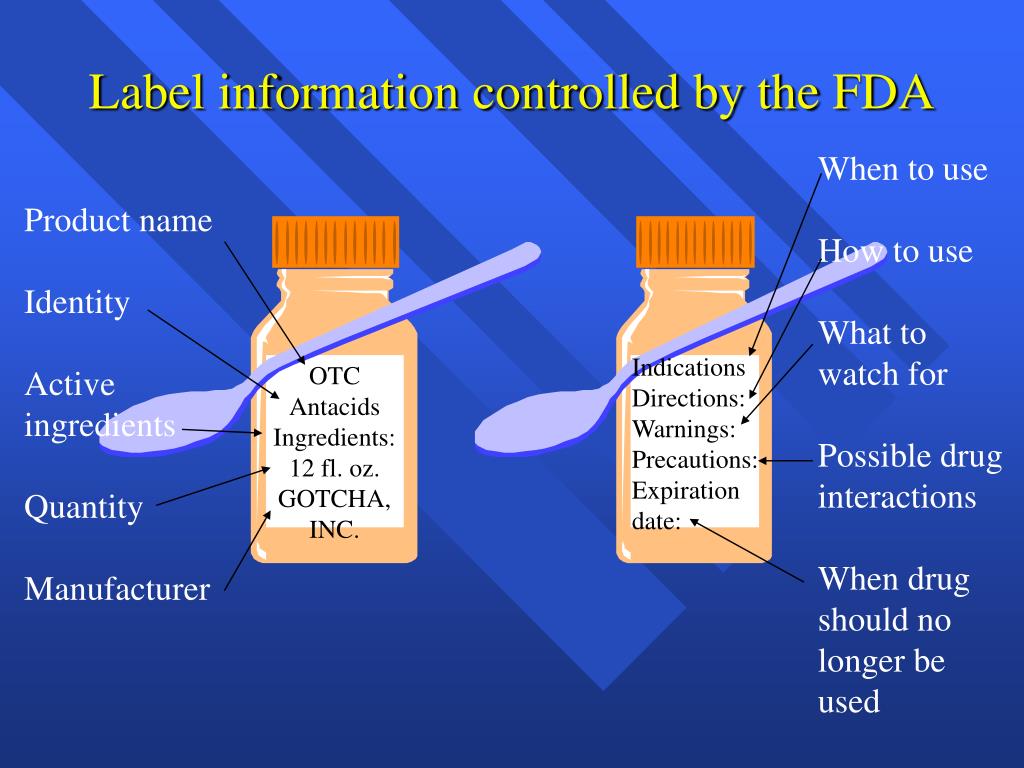
PPT Chapter 16 OvertheCounter (OTC) and Prescription Drugs
Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. search for labels on dailymed. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. Prescription drug labeling described in § 201.100(d) must meet the following. This paragraph applies only to prescription drug. (a) general requirements. (d) labeling requirements for new and more recently approved prescription drug products. The labels are also available on the national library of medicine's dailymed web site. for more information on labeling, including physician labeling rule (plr) requirements, guidances,.
From www.greenlight.guru
FDA Labeling Requirements Checklist Free Download Fda Labeling Requirements For Medications (d) labeling requirements for new and more recently approved prescription drug products. (a) general requirements. Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. in the united states,. Fda Labeling Requirements For Medications.
From www.icoptix.com
Labeling Laws/FDA and EU Guidance IC Optix Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug products. (a) general requirements. for more information on labeling, including physician labeling rule (plr) requirements,. Fda Labeling Requirements For Medications.
From www.youtube.com
How to read a medication label YouTube Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. (a) general requirements. search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug. Fda Labeling Requirements For Medications.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Labeling Requirements For Medications search for labels on dailymed. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. This paragraph applies only to prescription drug. (a) general requirements. (d) labeling requirements for new. Fda Labeling Requirements For Medications.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Labeling Requirements For Medications The labels are also available on the national library of medicine's dailymed web site. search for labels on dailymed. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the.. Fda Labeling Requirements For Medications.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Labeling Requirements For Medications The labels are also available on the national library of medicine's dailymed web site. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. This paragraph applies only to prescription drug. in the united states, the food and drug administration. Fda Labeling Requirements For Medications.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Labeling Requirements For Medications (a) general requirements. This paragraph applies only to prescription drug. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. (d) labeling requirements for new and more recently approved prescription drug products. The labels are also available on the national library of medicine's dailymed web site. Prescription drug labeling described in §. Fda Labeling Requirements For Medications.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Labeling Requirements For Medications search for labels on dailymed. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. (d) labeling requirements for new and more recently approved prescription drug products. The labels are also available on the national library of medicine's dailymed web site. (a) general. Fda Labeling Requirements For Medications.
From www.fdabasics.com
An Overview of FDA Requirements for OTC Drugs (Over the Counter Fda Labeling Requirements For Medications (a) general requirements. (d) labeling requirements for new and more recently approved prescription drug products. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. Prescription drug labeling. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications (a) general requirements. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. search for labels on dailymed. (d) labeling requirements for new and. Fda Labeling Requirements For Medications.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Labeling Requirements For Medications The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug products. This paragraph applies only to prescription drug. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. human prescription drug labeling (1) contains a summary of the essential scientific. Fda Labeling Requirements For Medications.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Labeling Requirements For Medications This paragraph applies only to prescription drug. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. human prescription drug labeling (1) contains a summary of the essential scientific information needed for.. Fda Labeling Requirements For Medications.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Labeling Requirements For Medications for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing. Fda Labeling Requirements For Medications.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Labeling Requirements For Medications This paragraph applies only to prescription drug. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. (d) labeling requirements for new and more recently approved prescription drug products. Prescription drug labeling described in § 201.100(d) must meet the following. in the united states, the food and drug administration (fda) is responsible. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications This paragraph applies only to prescription drug. Prescription drug labeling described in § 201.100(d) must meet the following. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. The labels. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications (d) labeling requirements for new and more recently approved prescription drug products. (a) general requirements. Prescription drug labeling described in § 201.100(d) must meet the following. This paragraph applies only to prescription drug. The labels are also available on the national library of medicine's dailymed web site. in the united states, the food and drug administration (fda). Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications (a) general requirements. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. search for labels on dailymed. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. human prescription drug labeling (1) contains a summary of the essential scientific. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications search for labels on dailymed. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. (a) general requirements. (d). Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications human prescription drug labeling (1) contains a summary of the essential scientific information needed for. The labels are also available on the national library of medicine's dailymed web site. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. (d) labeling requirements for new. Fda Labeling Requirements For Medications.
From rookqs.com
FDA Labeling Requirements for Devices Key Guide Fda Labeling Requirements For Medications (a) general requirements. search for labels on dailymed. (d) labeling requirements for new and more recently approved prescription drug products. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the.. Fda Labeling Requirements For Medications.
From www.slideserve.com
PPT Chapter 16 OvertheCounter (OTC) and Prescription Drugs Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. (d) labeling requirements for new and more recently approved prescription drug products. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. The labels are also available on the. Fda Labeling Requirements For Medications.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements For Medications in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. Prescription drug labeling described in § 201.100(d) must meet the following. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. (a) general requirements. (d) labeling requirements for. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications This paragraph applies only to prescription drug. Prescription drug labeling described in § 201.100(d) must meet the following. (a) general requirements. The labels are also available on the national library of medicine's dailymed web site. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. search for labels on dailymed. for. Fda Labeling Requirements For Medications.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Labeling Requirements For Medications for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. search for labels on dailymed. (a) general requirements. Prescription drug labeling described in § 201.100(d) must meet the following. This paragraph applies only to prescription drug. (d) labeling requirements for. Fda Labeling Requirements For Medications.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Labeling Requirements For Medications (d) labeling requirements for new and more recently approved prescription drug products. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of. Fda Labeling Requirements For Medications.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements For Medications The labels are also available on the national library of medicine's dailymed web site. This paragraph applies only to prescription drug. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. Prescription drug labeling described in § 201.100(d) must meet the following. (d) labeling requirements for new and more recently approved prescription drug. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. This paragraph applies only to prescription drug. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. search. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. This paragraph applies only to prescription drug. human prescription drug labeling (1) contains a summary of the essential scientific information needed. Fda Labeling Requirements For Medications.
From www.dionlabel.com
Navigating FDA Labelling Requirements for CBD Products Fda Labeling Requirements For Medications (a) general requirements. This paragraph applies only to prescription drug. search for labels on dailymed. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. in the united states, the food and drug administration (fda) is responsible for. Fda Labeling Requirements For Medications.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug products. This paragraph applies only to prescription. Fda Labeling Requirements For Medications.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Labeling Requirements For Medications human prescription drug labeling (1) contains a summary of the essential scientific information needed for. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. (d) labeling requirements for new and more recently approved prescription drug products. (a) general requirements. search for labels on dailymed. The labels are also available on the national. Fda Labeling Requirements For Medications.
From e-startupindia.com
US FDA labelling requirements for medical devices EStartupIndia Fda Labeling Requirements For Medications The labels are also available on the national library of medicine's dailymed web site. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. Prescription drug labeling described in § 201.100(d) must meet the following. (a) general requirements. This paragraph applies only to prescription drug.. Fda Labeling Requirements For Medications.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements For Medications search for labels on dailymed. human prescription drug labeling (1) contains a summary of the essential scientific information needed for. The labels are also available on the national library of medicine's dailymed web site. (d) labeling requirements for new and more recently approved prescription drug products. Prescription drug labeling described in § 201.100(d) must meet the following.. Fda Labeling Requirements For Medications.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. This paragraph applies only to prescription drug. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. The labels are also available on the national library of medicine's dailymed web site. search for labels on dailymed. (d) labeling requirements for new and more recently. Fda Labeling Requirements For Medications.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Medications Prescription drug labeling described in § 201.100(d) must meet the following. for more information on labeling, including physician labeling rule (plr) requirements, guidances,. in the united states, the food and drug administration (fda) is responsible for regulating and enforcing drug labeling to protect and ensure the. The labels are also available on the national library of medicine's dailymed. Fda Labeling Requirements For Medications.