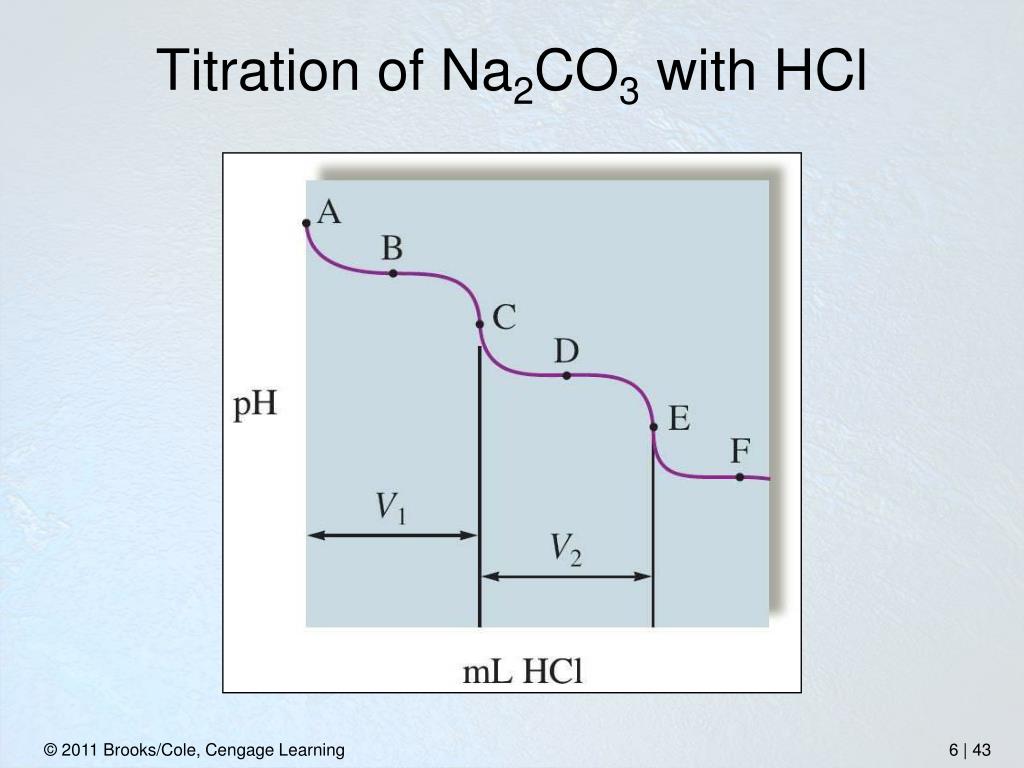
Titration Calculations Na2Co3 And Hcl . We apply the following formula, v 1 s 1 /v 2 s 2 = ½. From the balanced neutralization reaction equation, we get the following: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: 1 mole na 2 co 3 = 2 moles hcl. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The volume of the titrant.
from www.slideserve.com
1 mole na 2 co 3 = 2 moles hcl. We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. From the balanced neutralization reaction equation, we get the following: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The volume of the titrant.
PPT Fractions of H 2 CO 3 , HCO 3 , and CO 3 2 as a Function of pH
Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: 1 mole na 2 co 3 = 2 moles hcl. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The volume of the titrant. From the balanced neutralization reaction equation, we get the following: We apply the following formula, v 1 s 1 /v 2 s 2 = ½.
From childhealthpolicy.vumc.org
🌈 Na2co3 hcl titration. Why is used Na2CO3 with HCl in this titration Titration Calculations Na2Co3 And Hcl When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The volume of the titrant. From the balanced neutralization reaction equation, we get the following: The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The calculation for standardization. Titration Calculations Na2Co3 And Hcl.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: From the balanced neutralization reaction equation, we get the following: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate Titration Calculations Na2Co3 And Hcl We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The volume of the titrant. 1 mole na 2 co 3 = 2 moles hcl. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The calculation for standardization of hydrochloric acid with standard sodium carbonate. Titration Calculations Na2Co3 And Hcl.
From www.vrogue.co
Standardization Of Hcl Using Sodium Carbonate vrogue.co Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. 1 mole na 2. Titration Calculations Na2Co3 And Hcl.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Titration Calculations Na2Co3 And Hcl When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The volume of the titrant. In a titration. Titration Calculations Na2Co3 And Hcl.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The volume of the titrant. 1 mole na 2 co 3 = 2 moles. Titration Calculations Na2Co3 And Hcl.
From www.scribd.com
Na2CO3 HCL NaOH Titration PDF Redox Titration Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: 1 mole na 2 co 3 = 2 moles hcl. From the balanced neutralization reaction equation, we get the following: The volume of the titrant. Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m. Titration Calculations Na2Co3 And Hcl.
From www.chegg.com
Solved Lab 12 Acid Base Titration Report Part 1 Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. We apply the following formula, v 1 s 1. Titration Calculations Na2Co3 And Hcl.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Titration Calculations Na2Co3 And Hcl The volume of the titrant. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: From the balanced neutralization reaction equation, we get the following: When you add a hydrochloric acid (hcl) solution to. Titration Calculations Na2Co3 And Hcl.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Calculations Na2Co3 And Hcl We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The volume of the titrant. 1 mole na 2 co 3 = 2 moles hcl. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The calculation for standardization of hydrochloric acid with. Titration Calculations Na2Co3 And Hcl.
From www.slideserve.com
PPT Fractions of H 2 CO 3 , HCO 3 , and CO 3 2 as a Function of pH Titration Calculations Na2Co3 And Hcl The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: From the balanced neutralization reaction equation, we get the following: In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Titration curves for 50.0 ml of 1.0 × 10 −. Titration Calculations Na2Co3 And Hcl.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Titration Calculations Na2Co3 And Hcl 1 mole na 2 co 3 = 2 moles hcl. We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The volume of the titrant. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The calculation for standardization of hydrochloric acid with. Titration Calculations Na2Co3 And Hcl.
From www.tessshebaylo.com
Chemical Equation For Sodium Carbonate And Hydrochloric Acid Tessshebaylo Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The volume of the titrant. We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: In a titration of sulfuric acid. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide Titration Calculations Na2Co3 And Hcl We apply the following formula, v 1 s 1 /v 2 s 2 = ½. The volume of the titrant. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. 1 mole na 2 co 3 = 2 moles hcl. When you add a hydrochloric acid (hcl) solution to. Titration Calculations Na2Co3 And Hcl.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Titration Calculations Na2Co3 And Hcl We apply the following formula, v 1 s 1 /v 2 s 2 = ½. Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. The equation for the. Titration Calculations Na2Co3 And Hcl.
From tukioka-clinic.com
😂 Titration of naoh and na2co3 with hcl. Titration Of Hcl And Na2co3 Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous. Titration Calculations Na2Co3 And Hcl.
From www.meritnation.com
Solve this Q 49 In the titration of Na2CO3 by HCl using methyl orange Titration Calculations Na2Co3 And Hcl 1 mole na 2 co 3 = 2 moles hcl. Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. We apply the following formula, v 1 s 1. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
titration of sodium carbonate with HCI titration of Na2CO3 Vs HCI Titration Calculations Na2Co3 And Hcl When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube Titration Calculations Na2Co3 And Hcl In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown. Titration Calculations Na2Co3 And Hcl.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Titration Calculations Na2Co3 And Hcl The volume of the titrant. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Net Ionic Equation for Na2CO3 + HCl Sodium carbonate + Hydrochloric Titration Calculations Na2Co3 And Hcl Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. From the balanced neutralization reaction equation, we get the following: The volume of the titrant. The calculation for standardization. Titration Calculations Na2Co3 And Hcl.
From byjus.com
nt20 ml of 0.1 M solution of compound Na2CO3.NaHCO3.2H2O is titrated Titration Calculations Na2Co3 And Hcl 1 mole na 2 co 3 = 2 moles hcl. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: From the balanced neutralization reaction equation, we get the following:. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Na2CO3 vs HCl Titration CBSE Chemistry Practical Titration Class 11 Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: The volume of the titrant. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. Titration curves for 50.0 ml of 1.0 × 10. Titration Calculations Na2Co3 And Hcl.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Titration Calculations Na2Co3 And Hcl The volume of the titrant. When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below. Titration Calculations Na2Co3 And Hcl.
From childhealthpolicy.vumc.org
🌈 Na2co3 hcl titration. Why is used Na2CO3 with HCl in this titration Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. We apply the following formula, v 1 s 1 /v 2 s 2 = ½. Titration curves for 50.0 ml of 1.0 ×. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
How to Balance Na2CO3 + HCl = NaCl + H2O + CO2 (Sodium carbonate Titration Calculations Na2Co3 And Hcl Titration curves for 50.0 ml of 1.0 × 10 − 4 m hcl using 1.0 × 10 − 4 m naoh in (a) water, kw = 1.0 × 10 − 14, and (b) a nonaqueous amphiprotic solvent, ks = 1.0 ×. From the balanced neutralization reaction equation, we get the following: The calculation for standardization of hydrochloric acid with standard. Titration Calculations Na2Co3 And Hcl.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Calculations Na2Co3 And Hcl In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The volume of the titrant. 1 mole na 2 co 3 = 2 moles hcl. From the balanced neutralization reaction equation, we get the. Titration Calculations Na2Co3 And Hcl.
From boys.velvet.jp
Net Ionic Equation For Na2CO3 HCl Sodium Carbonate, 42 OFF Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: 1 mole na 2 co 3 = 2 moles hcl. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: From the balanced neutralization reaction equation, we get the following: The volume of the titrant. Titration. Titration Calculations Na2Co3 And Hcl.
From www.chegg.com
Solved Experiment (?)B Standardization of HCl with Na2CO3 Titration Calculations Na2Co3 And Hcl From the balanced neutralization reaction equation, we get the following: The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. The equation for the second stage of the reaction between sodium carbonate and hydrochloric. Titration Calculations Na2Co3 And Hcl.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Calculations Na2Co3 And Hcl The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. 1 mole na 2 co 3 = 2 moles hcl. In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Calculations Na2Co3 And Hcl 1 mole na 2 co 3 = 2 moles hcl. The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: We apply the following formula, v 1 s 1 /v 2 s 2 = ½. From the balanced neutralization reaction equation, we get the following: In a titration of sulfuric acid. Titration Calculations Na2Co3 And Hcl.
From www.numerade.com
SOLVED 'Determination the normality of a sodium hydroxide solution Titration Calculations Na2Co3 And Hcl The volume of the titrant. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: In a titration of sulfuric acid against sodium hydroxide, 32.20ml of 0.250mnaoh is required to neutralize 26.60ml of h 2so 4. 1 mole na 2 co 3 = 2 moles hcl. From the balanced neutralization reaction equation, we get the. Titration Calculations Na2Co3 And Hcl.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Calculations Na2Co3 And Hcl The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: When you add a hydrochloric acid (hcl) solution to a solution of sodium carbonate (na 2 co 3), the hydrogen ion in. Titration curves for 50.0. Titration Calculations Na2Co3 And Hcl.
From www.chegg.com
Solved 1. Consider the titration of Na2CO3 with HCl to Titration Calculations Na2Co3 And Hcl The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: From the balanced neutralization reaction equation, we get the following: We apply the following formula, v 1 s 1 /v 2 s 2 = ½. In. Titration Calculations Na2Co3 And Hcl.
From www.youtube.com
Lab 2 (Titration of HCl with Na2CO3) YouTube Titration Calculations Na2Co3 And Hcl 1 mole na 2 co 3 = 2 moles hcl. The calculation for standardization of hydrochloric acid with standard sodium carbonate solution is given below: The equation for the second stage of the reaction between sodium carbonate and hydrochloric acid is shown below again: The volume of the titrant. From the balanced neutralization reaction equation, we get the following: We. Titration Calculations Na2Co3 And Hcl.