What Are Colloid And Solution . Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A solution is a homogeneous mixture of two or more components. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. The substance that is dissolved is. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids consist of two distinct phases: The dissolving agent is the solvent. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. These particles can be solid, liquid, or gas.
from www.theplasticsfella.com
Unlike true solutions where solute. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The substance that is dissolved is. Colloids consist of two distinct phases: The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A solution is a homogeneous mixture of two or more components. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely.
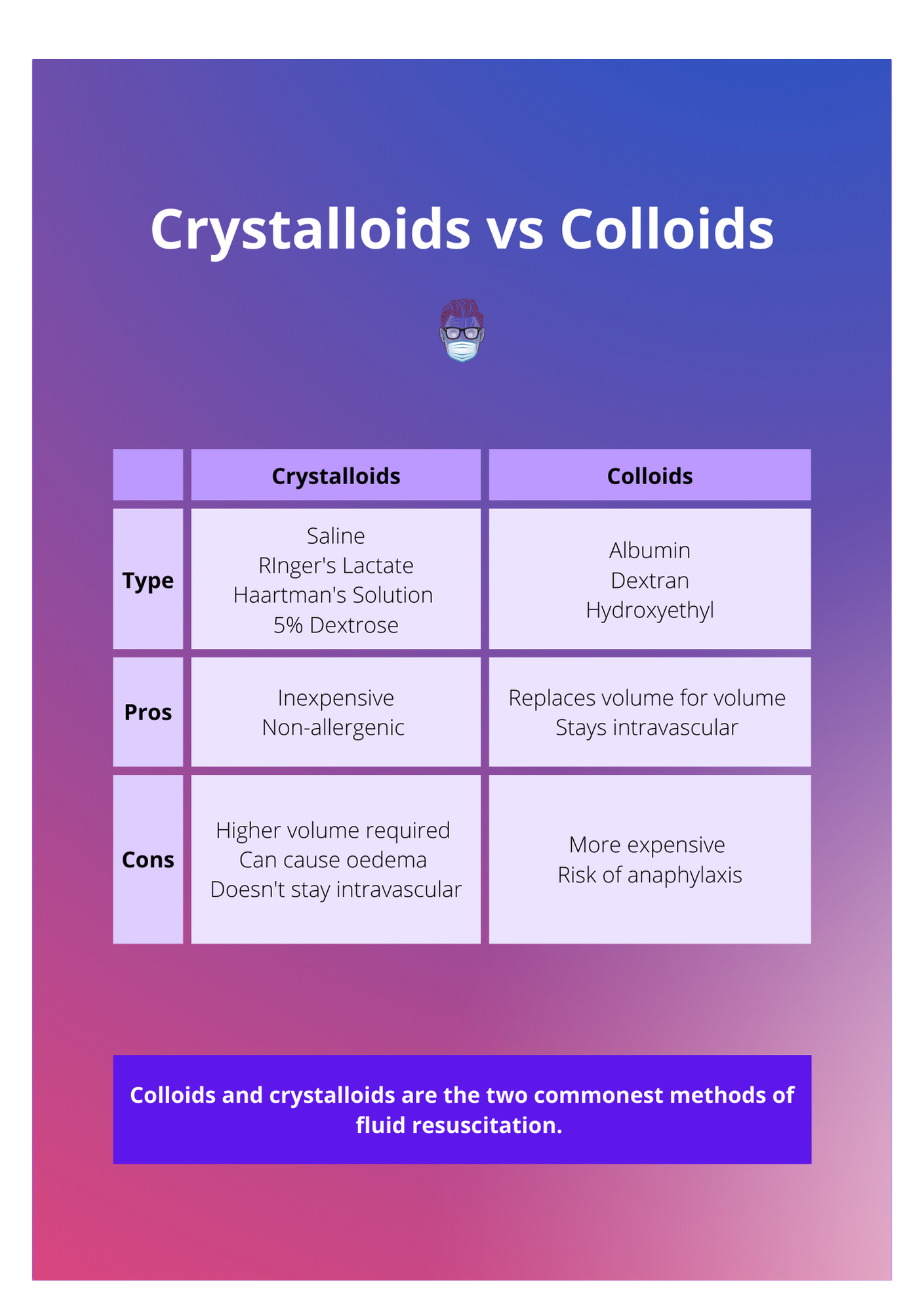
Fluid Resuscitation in Burns · Formulas, Indications & Fluids
What Are Colloid And Solution These particles can be solid, liquid, or gas. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloids consist of two distinct phases: In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can be solid, liquid, or gas. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The dissolving agent is the solvent. A solution is a homogeneous mixture of two or more components. The substance that is dissolved is. Unlike true solutions where solute.
From 1svoimi-rukami.ru
Как сделать коллоидный раствор дома 82 фото What Are Colloid And Solution In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The dissolving agent is the solvent. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids consist of. What Are Colloid And Solution.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference What Are Colloid And Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The substance that is dissolved is. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. Unlike true solutions where solute. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids consist of two. What Are Colloid And Solution.
From webapi.bu.edu
Properties of colloidal solution. Colloidal System. 20221020 What Are Colloid And Solution Unlike true solutions where solute. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids consist of two distinct phases: In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A. What Are Colloid And Solution.
From www.slideshare.net
Colloids What Are Colloid And Solution Colloids consist of two distinct phases: These particles can be solid, liquid, or gas. The substance that is dissolved is. A solution is a homogeneous mixture of two or more components. Unlike true solutions where solute. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The particles are microscopic in size, ranging from. What Are Colloid And Solution.
From pt.slideshare.net
Solutions, suspensions, and colloids What Are Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloids consist of two distinct phases: The substance that is dissolved is. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. These particles can be solid, liquid, or gas. A solution is a homogeneous. What Are Colloid And Solution.
From ar.inspiredpencil.com
Examples Of Colloid Mixtures What Are Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Colloids consist of two distinct phases: The substance that is dissolved is. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Unlike true solutions where solute. In addition, we will discuss colloids—systems that resemble. What Are Colloid And Solution.
From www.toppr.com
Which of the following is/are a type of homogenous mixture? What Are Colloid And Solution The dissolving agent is the solvent. These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloid is. What Are Colloid And Solution.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download What Are Colloid And Solution The substance that is dissolved is. Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The dissolving agent is the solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between. What Are Colloid And Solution.
From www.theplasticsfella.com
Fluid Resuscitation in Burns · Formulas, Indications & Fluids What Are Colloid And Solution Colloids consist of two distinct phases: Unlike true solutions where solute. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. These particles can be solid, liquid, or gas. A solution is a homogeneous mixture of two or more components. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In. What Are Colloid And Solution.
From edurev.in
Difference between true solution, suspension and colloidal sol What Are Colloid And Solution These particles can be solid, liquid, or gas. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The dissolving agent is the solvent. Colloids consist of two distinct phases: The dispersed phase (particles) and. What Are Colloid And Solution.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions What Are Colloid And Solution These particles can be solid, liquid, or gas. The dissolving agent is the solvent. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The substance that is dissolved is. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. A colloid is a heterogeneous mixture in which. What Are Colloid And Solution.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube What Are Colloid And Solution These particles can be solid, liquid, or gas. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Unlike true solutions where solute. A. What Are Colloid And Solution.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's What Are Colloid And Solution The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. The dissolving agent is the solvent. Colloids consist of two distinct phases: Unlike true solutions where solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloidal solution typically consists of particles ranging in size. What Are Colloid And Solution.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download What Are Colloid And Solution In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Colloids consist of two distinct phases: A solution is a homogeneous mixture of two or more components. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. These particles can be solid, liquid, or gas. Unlike true solutions. What Are Colloid And Solution.
From sciencenotes.org
What Is a Colloid? Definition and Examples What Are Colloid And Solution Colloids consist of two distinct phases: Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture in which the. What Are Colloid And Solution.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz What Are Colloid And Solution The dissolving agent is the solvent. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Unlike true solutions where solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. These particles can be solid, liquid, or gas. A colloidal solution typically consists of. What Are Colloid And Solution.
From www.slideserve.com
PPT COLLOIDS PowerPoint Presentation, free download ID4101356 What Are Colloid And Solution A solution is a homogeneous mixture of two or more components. The substance that is dissolved is. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Unlike true solutions where solute. In chemistry, a colloid is a. What Are Colloid And Solution.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download What Are Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. Colloids consist of two distinct phases: A colloidal solution typically consists of. What Are Colloid And Solution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID What Are Colloid And Solution The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. The substance that is dissolved is. Unlike true solutions where solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer.. What Are Colloid And Solution.
From foodsciencehm.blogspot.com
Food Science Notes for 2nd Semester HM students UNIT 8 COLLOIDS What Are Colloid And Solution Colloids consist of two distinct phases: Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components. The substance that is dissolved is. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer.. What Are Colloid And Solution.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of What Are Colloid And Solution The substance that is dissolved is. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. These particles can be solid, liquid, or gas. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1.. What Are Colloid And Solution.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True What Are Colloid And Solution These particles can be solid, liquid, or gas. The dissolving agent is the solvent. The substance that is dissolved is. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components. In chemistry, a colloid is a mixture of tiny particles. What Are Colloid And Solution.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru What Are Colloid And Solution Colloids consist of two distinct phases: A solution is a homogeneous mixture of two or more components. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely.. What Are Colloid And Solution.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download What Are Colloid And Solution Unlike true solutions where solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. Colloids consist of two distinct phases: The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. These particles can. What Are Colloid And Solution.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples What Are Colloid And Solution The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. A solution is a homogeneous mixture of two or more components. Colloids consist of two distinct phases: A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. The substance that is dissolved. What Are Colloid And Solution.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation What Are Colloid And Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a colloid is a mixture of tiny particles that are dispersed in another medium. The substance that is dissolved is. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. A solution is a homogeneous mixture of two. What Are Colloid And Solution.
From uen.pressbooks.pub
Colloids Introductory Chemistry What Are Colloid And Solution The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. The substance that is dissolved is. Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles. What Are Colloid And Solution.
From chemistrytalk.org
What Are Colloids? ChemTalk What Are Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. Colloids consist of two distinct phases: In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. The particles are. What Are Colloid And Solution.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion What Are Colloid And Solution A solution is a homogeneous mixture of two or more components. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Unlike true solutions where solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The substance that is dissolved is. The dissolving agent. What Are Colloid And Solution.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension What Are Colloid And Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. Colloids consist of two distinct phases: The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A solution is a homogeneous mixture of two or more components. A colloid is a heterogeneous mixture. What Are Colloid And Solution.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free What Are Colloid And Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. The substance that is dissolved is. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. In chemistry, a. What Are Colloid And Solution.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs What Are Colloid And Solution A solution is a homogeneous mixture of two or more components. These particles can be solid, liquid, or gas. In addition, we will discuss colloids—systems that resemble solutions but consist of dispersions of particles somewhat. Unlike true solutions where solute. The substance that is dissolved is. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The. What Are Colloid And Solution.
From chemistnotes.com
Colloids Definition, Examples, Properties, and 10 Reliable What Are Colloid And Solution A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. These particles can be solid, liquid, or gas. Unlike true solutions where solute. Colloids consist of two. What Are Colloid And Solution.
From www.youtube.com
Solution Suspension Colloid YouTube What Are Colloid And Solution The particles are microscopic in size, ranging from 1 nanometer (nm) to 1. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. These particles can be solid, liquid, or gas. The dissolving agent is the solvent. A solution is a homogeneous mixture of two or more components. The substance that. What Are Colloid And Solution.
From www.animalia-life.club
Examples Of Colloids Mixtures What Are Colloid And Solution The dissolving agent is the solvent. The dispersed phase (particles) and the continuous phase (medium), which coexist without dissolving completely. A colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. Unlike true solutions where solute. A solution is a homogeneous mixture of two or more components.. What Are Colloid And Solution.