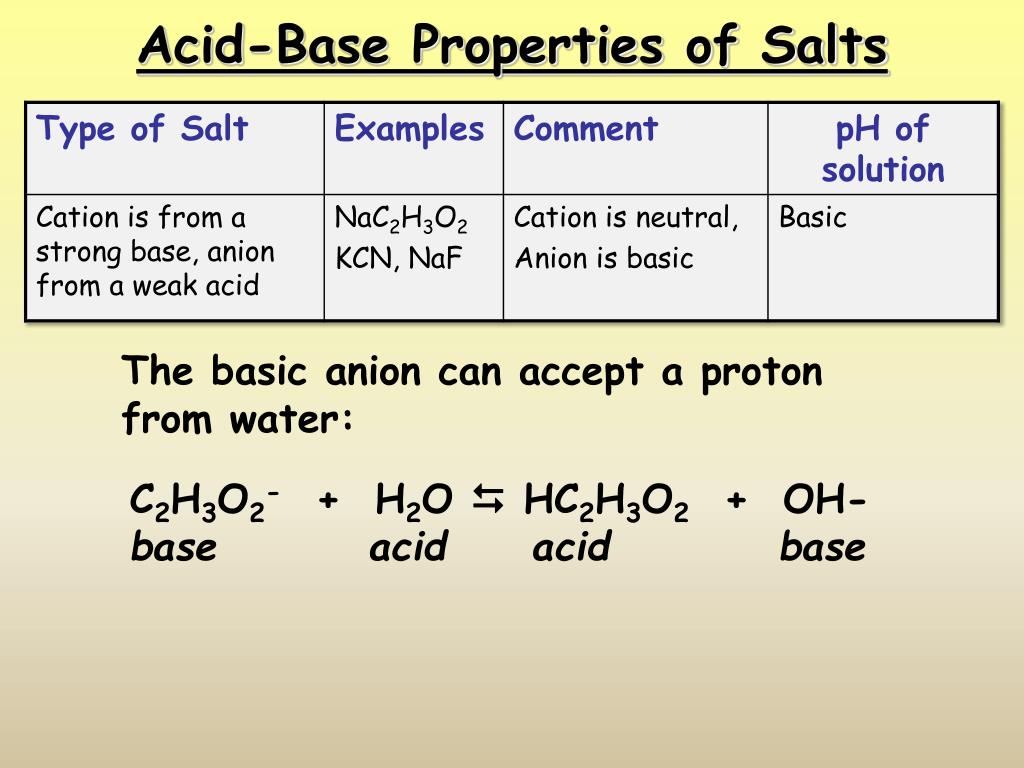
What Are The Characteristics Of Acid Base And Salt . A base is a molecule or ion able to accept a hydrogen ion from an acid. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Acidic substances are usually identified by their sour taste. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. An acid is basically a molecule which can. The ions of the salt are the conjugate base and conjugate acid of the acid. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Salts can be neutral, acidic or basic. Bases, on the other hand, are substances that accept protons or donate. How do the k a or k b.
from www.slideserve.com
Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Salts can be neutral, acidic or basic. An acid is basically a molecule which can. The ions of the salt are the conjugate base and conjugate acid of the acid. Acidic substances are usually identified by their sour taste. Bases, on the other hand, are substances that accept protons or donate. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. How do the k a or k b. A base is a molecule or ion able to accept a hydrogen ion from an acid.
PPT AcidBase Properties of Salts PowerPoint Presentation, free
What Are The Characteristics Of Acid Base And Salt A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. A base is a molecule or ion able to accept a hydrogen ion from an acid. How do the k a or k b. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Salts can be neutral, acidic or basic. An acid is basically a molecule which can. Acidic substances are usually identified by their sour taste. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. Bases, on the other hand, are substances that accept protons or donate. The ions of the salt are the conjugate base and conjugate acid of the acid.
From chemistry.analia-sanchez.net
Acidbasessalt Notes Chemistry Classes / Ronald Reagan S.H.S. What Are The Characteristics Of Acid Base And Salt Acidic substances are usually identified by their sour taste. How do the k a or k b. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Salts can be neutral, acidic or basic. Bases, on the other hand, are substances that. What Are The Characteristics Of Acid Base And Salt.
From studylistlangdon.z21.web.core.windows.net
Properties Of Acid And Bases Worksheet What Are The Characteristics Of Acid Base And Salt Bases, on the other hand, are substances that accept protons or donate. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. A salt can dissolve in water. What Are The Characteristics Of Acid Base And Salt.
From www.youtube.com
Acid, Bases and Salts Part I Definitions and Classification of Salts What Are The Characteristics Of Acid Base And Salt Bases, on the other hand, are substances that accept protons or donate. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a. What Are The Characteristics Of Acid Base And Salt.
From www.successcds.net
Acids Bases and Salts Class 10 Notes Science Chapter 2 Explanation What Are The Characteristics Of Acid Base And Salt Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. Salts can be neutral, acidic or basic. Acidic substances are usually identified by their sour taste. Bases, on the other hand, are substances that accept protons or donate. A salt can dissolve in water to. What Are The Characteristics Of Acid Base And Salt.
From www.studypool.com
SOLUTION The Fundamentals of AcidBase and Salt Chemistry An InDepth What Are The Characteristics Of Acid Base And Salt The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. How do the k a or k b. Acidic substances are usually identified by their sour taste. The ions of the salt are the conjugate base and conjugate acid of the acid. Salts can be neutral, acidic or basic. A salt can dissolve. What Are The Characteristics Of Acid Base And Salt.
From www.slideserve.com
PPT ACIDS, BASES AND SALTS PowerPoint Presentation, free download What Are The Characteristics Of Acid Base And Salt A base is a molecule or ion able to accept a hydrogen ion from an acid. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Salts can be neutral, acidic or basic. The properties of acids include a sour taste, ability. What Are The Characteristics Of Acid Base And Salt.
From learningschoolegestive.z5.web.core.windows.net
The Properties Of Acids And Bases What Are The Characteristics Of Acid Base And Salt Salts can be neutral, acidic or basic. Bases, on the other hand, are substances that accept protons or donate. The ions of the salt are the conjugate base and conjugate acid of the acid. How do the k a or k b. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on. What Are The Characteristics Of Acid Base And Salt.
From studylib.net
Acid Base Properties of Salts 2013 What Are The Characteristics Of Acid Base And Salt Bases, on the other hand, are substances that accept protons or donate. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. A. What Are The Characteristics Of Acid Base And Salt.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Are The Characteristics Of Acid Base And Salt An acid is basically a molecule which can. A base is a molecule or ion able to accept a hydrogen ion from an acid. Bases, on the other hand, are substances that accept protons or donate. The ions of the salt are the conjugate base and conjugate acid of the acid. The properties of acids include a sour taste, ability. What Are The Characteristics Of Acid Base And Salt.
From www.slideserve.com
PPT What are some characteristics of acids and bases? PowerPoint What Are The Characteristics Of Acid Base And Salt How do the k a or k b. An acid is basically a molecule which can. A base is a molecule or ion able to accept a hydrogen ion from an acid. The ions of the salt are the conjugate base and conjugate acid of the acid. A salt can dissolve in water to produce a neutral, a basic, or. What Are The Characteristics Of Acid Base And Salt.
From www.sssi.in
Acids, Bases, and Salts Key Concepts and Properties Explained What Are The Characteristics Of Acid Base And Salt Bases, on the other hand, are substances that accept protons or donate. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? An acid is basically a molecule which can. A. What Are The Characteristics Of Acid Base And Salt.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt What Are The Characteristics Of Acid Base And Salt A base is a molecule or ion able to accept a hydrogen ion from an acid. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Bases, on the other hand, are substances that accept protons or donate. Why does a salt containing a cation from a weak base and an anion from. What Are The Characteristics Of Acid Base And Salt.
From classlibjeske.z21.web.core.windows.net
List The Properties Of Acids And Bases What Are The Characteristics Of Acid Base And Salt Salts can be neutral, acidic or basic. The ions of the salt are the conjugate base and conjugate acid of the acid. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. A salt can dissolve in water to produce a neutral, a basic, or. What Are The Characteristics Of Acid Base And Salt.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets What Are The Characteristics Of Acid Base And Salt Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? The ions of the salt are the conjugate base and conjugate acid of the acid. A base is a molecule or ion able to accept a hydrogen ion from an acid. Bases (or alkalies) were characterized mainly by. What Are The Characteristics Of Acid Base And Salt.
From learningschoolvocalist.z14.web.core.windows.net
List The Properties Of Acids And Bases What Are The Characteristics Of Acid Base And Salt Salts can be neutral, acidic or basic. Bases, on the other hand, are substances that accept protons or donate. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. An acid is basically a molecule which can. Acidic substances are usually identified by their sour. What Are The Characteristics Of Acid Base And Salt.
From www.slideserve.com
PPT Intro to Acids, Bases, & Salts PowerPoint Presentation, free What Are The Characteristics Of Acid Base And Salt The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Salts can be neutral, acidic or basic. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Acidic substances are usually identified by their sour taste. A salt can dissolve in. What Are The Characteristics Of Acid Base And Salt.
From www.slideserve.com
PPT ACID, BASE, SALT (Including Atom, Ion, Molecules) PowerPoint What Are The Characteristics Of Acid Base And Salt A base is a molecule or ion able to accept a hydrogen ion from an acid. How do the k a or k b. Acidic substances are usually identified by their sour taste. Bases, on the other hand, are substances that accept protons or donate. A salt can dissolve in water to produce a neutral, a basic, or an acidic. What Are The Characteristics Of Acid Base And Salt.
From www.onlinenotesnepal.com
Acid, Base, and Salt in Class 10 Science Online Notes Nepal What Are The Characteristics Of Acid Base And Salt Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. Acidic substances are usually identified by their sour taste. Salts can be neutral, acidic or basic. How do the k a or k b. Bases, on the other hand, are substances that accept protons or. What Are The Characteristics Of Acid Base And Salt.
From byjus.com
Class 10 Chapter 2 Science Notes Acids, Bases, and Salts What Are The Characteristics Of Acid Base And Salt Bases, on the other hand, are substances that accept protons or donate. Acidic substances are usually identified by their sour taste. How do the k a or k b. An acid is basically a molecule which can. Salts can be neutral, acidic or basic. The ions of the salt are the conjugate base and conjugate acid of the acid. The. What Are The Characteristics Of Acid Base And Salt.
From www.yourdictionary.com
Difference Between Acids and Bases Key Properties YourDictionary What Are The Characteristics Of Acid Base And Salt Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. A base is a molecule or ion able to accept a hydrogen ion from an acid. Salts can be neutral, acidic or basic. Why does a salt containing a cation from a weak base and. What Are The Characteristics Of Acid Base And Salt.
From www.dreamstime.com
Acids Bases and Salts Infographic Diagram Stock Vector Illustration What Are The Characteristics Of Acid Base And Salt An acid is basically a molecule which can. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. A base is a molecule or ion able to accept a hydrogen ion from an acid. How do the k a or k b. Acidic substances are. What Are The Characteristics Of Acid Base And Salt.
From 88guru.com
What are Salts in Chemistry Characteristics, Types 88guru What Are The Characteristics Of Acid Base And Salt The ions of the salt are the conjugate base and conjugate acid of the acid. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. A base is a molecule or ion able to accept a hydrogen ion from an acid. How do the k a or k b. Bases, on the other. What Are The Characteristics Of Acid Base And Salt.
From www.youtube.com
Acid Bases Salts YouTube What Are The Characteristics Of Acid Base And Salt Acidic substances are usually identified by their sour taste. Bases, on the other hand, are substances that accept protons or donate. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. An acid is basically a molecule which can. Salts can be neutral, acidic or. What Are The Characteristics Of Acid Base And Salt.
From slideplayer.com
Acids, Bases & Salts. ppt download What Are The Characteristics Of Acid Base And Salt A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Bases, on the other hand, are substances that accept protons or donate. The ions of the salt are the conjugate base and conjugate acid of the acid. Bases (or alkalies) were characterized. What Are The Characteristics Of Acid Base And Salt.
From www.youtube.com
Acidbase properties of salts Acids and bases AP Chemistry Khan What Are The Characteristics Of Acid Base And Salt An acid is basically a molecule which can. Acidic substances are usually identified by their sour taste. Bases, on the other hand, are substances that accept protons or donate. The ions of the salt are the conjugate base and conjugate acid of the acid. How do the k a or k b. The properties of acids include a sour taste,. What Are The Characteristics Of Acid Base And Salt.
From pediaa.com
Difference Between Acid and Base What Are The Characteristics Of Acid Base And Salt Acidic substances are usually identified by their sour taste. An acid is basically a molecule which can. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Salts can be neutral, acidic or basic. A base is a molecule or ion able to accept a hydrogen ion from an acid. A salt can. What Are The Characteristics Of Acid Base And Salt.
From www.vrogue.co
Acids Bases And Salts Class 10 Chapter 1 Short Notes vrogue.co What Are The Characteristics Of Acid Base And Salt How do the k a or k b. Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Why does a salt containing a cation from a weak. What Are The Characteristics Of Acid Base And Salt.
From slidetodoc.com
Acids Bases Salts Characteristic Properties of Acids and What Are The Characteristics Of Acid Base And Salt The ions of the salt are the conjugate base and conjugate acid of the acid. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? How do the k a or k b. Salts can be neutral, acidic or basic. The properties of acids include a sour taste,. What Are The Characteristics Of Acid Base And Salt.
From www.slideserve.com
PPT AcidBase Properties of Salts PowerPoint Presentation, free What Are The Characteristics Of Acid Base And Salt The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Bases, on the other hand, are substances that accept protons or donate. Acidic substances are usually identified by their sour taste. The ions of the salt are the conjugate base and conjugate acid of the acid. A base is a molecule or ion. What Are The Characteristics Of Acid Base And Salt.
From www.sliderbase.com
AcidBase Properties of Salts Presentation Chemistry What Are The Characteristics Of Acid Base And Salt The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. How do the k a or k b. An acid is basically a molecule which can. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? The ions of the salt. What Are The Characteristics Of Acid Base And Salt.
From slideplayer.com
Acids, Bases & Salts. ppt download What Are The Characteristics Of Acid Base And Salt The ions of the salt are the conjugate base and conjugate acid of the acid. An acid is basically a molecule which can. How do the k a or k b. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Salts can be neutral, acidic or basic. A base is a molecule. What Are The Characteristics Of Acid Base And Salt.
From ifunny.co
How Section 3 of 4 Differences Between Acids and Bases characteristic What Are The Characteristics Of Acid Base And Salt Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Bases, on the other hand, are substances. What Are The Characteristics Of Acid Base And Salt.
From brainly.in
chemical properties of acids, bases and salt Brainly.in What Are The Characteristics Of Acid Base And Salt A salt can dissolve in water to produce a neutral, a basic, or an acidic solution, depending on whether it contains the conjugate base of a weak acid. Why does a salt containing a cation from a weak base and an anion from a strong acid form an acidic solution? Bases, on the other hand, are substances that accept protons. What Are The Characteristics Of Acid Base And Salt.
From www.youtube.com
Acids & Bases Acid Base Properties of Salts. YouTube What Are The Characteristics Of Acid Base And Salt How do the k a or k b. An acid is basically a molecule which can. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. Salts can be neutral, acidic or basic. The ions of the salt are the conjugate base and conjugate acid of the acid. Why does a salt containing. What Are The Characteristics Of Acid Base And Salt.
From dokumen.tips
(PDF) STUDENT VERSION Unit 9 Acids, Bases, & · PDF file... Acids What Are The Characteristics Of Acid Base And Salt Bases (or alkalies) were characterized mainly by their ability to neutralize acids and form salts, the latter being typified rather loosely as crystalline substances soluble. Acidic substances are usually identified by their sour taste. An acid is basically a molecule which can. The properties of acids include a sour taste, ability to turn blue litmus paper red, and corrosiveness. The. What Are The Characteristics Of Acid Base And Salt.