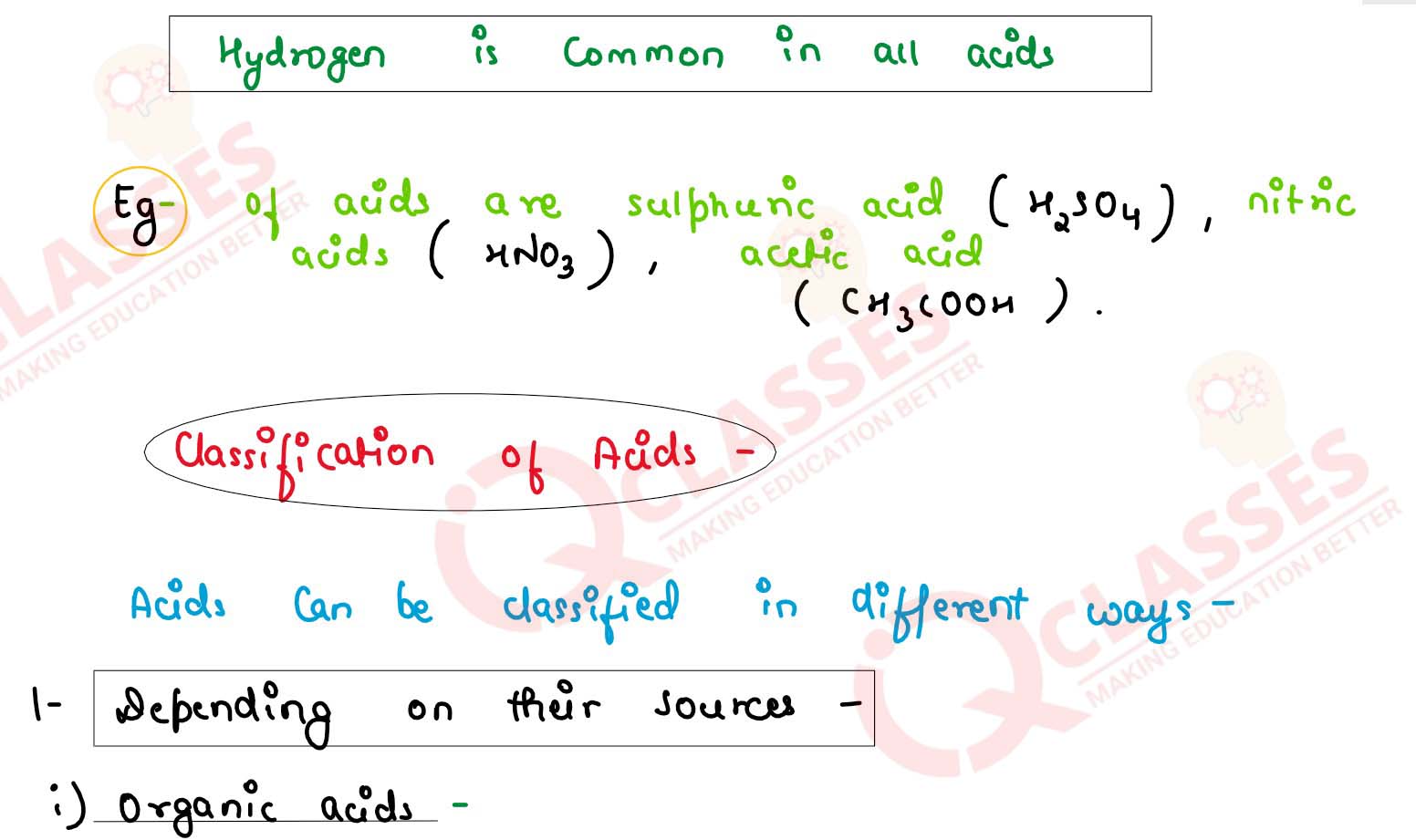
Bases Definition Class 10 . In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. In this post, we will discuss the. Both react to form salts and water in. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. Dilution of acid and base: Usually, the acidic substances are identified with their sour taste. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. A base is a molecule or ion able to accept a hydrogen ion from an acid. An acid is basically a molecule which can. Acidic substances are usually identified by their sour taste. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue.
from iqclasses.in
In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. In this post, we will discuss the. An acid is basically a molecule which can. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. Usually, the acidic substances are identified with their sour taste. Dilution of acid and base: While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Both react to form salts and water in. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue.
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts
Bases Definition Class 10 A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Usually, the acidic substances are identified with their sour taste. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. Both react to form salts and water in. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. An acid is basically a molecule which can. In this post, we will discuss the. A base is a molecule or ion able to accept a hydrogen ion from an acid. Dilution of acid and base: Acidic substances are usually identified by their sour taste. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and.
From www.youtube.com
Class 10 Chemistry Chapter 3 Acids & Bases/ Definition, Strong & Weak Bases Definition Class 10 Usually, the acidic substances are identified with their sour taste. An acid is basically a molecule which can. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. Both react. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. In this post, we will discuss the. Usually, the acidic substances are identified with their sour taste. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. While we. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Usually, the acidic substances are identified with their sour taste. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. The concentration of hydrogen ion in an acid and hydroxide. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Acidic substances are usually identified by their sour taste. A base is a molecule or ion able to accept a hydrogen ion from an acid. Dilution of acid and base: The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. In class 10 chapter 2 notes, students learn about the difference between. Bases Definition Class 10.
From exykwhjcv.blob.core.windows.net
What Is Meaning Of Base In English at Alisha Myers blog Bases Definition Class 10 In this post, we will discuss the. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Acidic substances are usually identified by their sour taste. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. Dilution of. Bases Definition Class 10.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Bases Definition Class 10 Dilution of acid and base: In this post, we will discuss the. Acidic substances are usually identified by their sour taste. An acid is basically a molecule which can. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. Usually, the acidic substances are identified with their sour. Bases Definition Class 10.
From www.sssi.in
Acids, Bases, and Salts Key Concepts and Properties Explained Bases Definition Class 10 A base is an ion or molecule that is able to accept a hydrogen ion from an acid. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. A base is a molecule or ion able to accept a hydrogen ion from an acid. Both react to form. Bases Definition Class 10.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples Bases Definition Class 10 The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. An acid is basically a molecule which can. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Dilution of acid and base: In class 10 chapter 2 notes, students. Bases Definition Class 10.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. In this post, we will discuss the. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Both react to form salts and water in. In class 10 chapter. Bases Definition Class 10.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Bases Definition Class 10 The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. A base is a molecule or ion able to accept a hydrogen ion from an acid. Both react to. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. Usually, the acidic substances are identified with their sour taste. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. In class 10 chapter 2 notes, students. Bases Definition Class 10.
From www.blendspace.com
Acids And Bases Introduction Lessons Blendspace Bases Definition Class 10 A base is a molecule or ion able to accept a hydrogen ion from an acid. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Usually, the. Bases Definition Class 10.
From www.tiwariacademy.com
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts Bases Definition Class 10 Dilution of acid and base: Usually, the acidic substances are identified with their sour taste. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. A base is a molecule or ion able to accept a hydrogen ion from an acid. The concentration of hydrogen ion in an. Bases Definition Class 10.
From www.youtube.com
Acid Bases and Salts Class 10 Science CBSE Chemical Properties of Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Usually, the acidic substances are identified with their sour taste. Both react to form salts and water in. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. An. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. Usually, the acidic substances are identified with their sour taste. A. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Dilution of acid and base: Both react to form salts and water in. Usually, the acidic substances are identified with their sour taste. An acid is basically a molecule which can. Acidic substances are usually identified by their sour taste. The concentration. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. An acid is basically a molecule which can. Acidic substances are usually identified by their sour taste. Both react to form salts and water in. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7,. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. Usually, the acidic substances are identified with their sour taste. In the field of chemistry, a ‘base’ can be. Bases Definition Class 10.
From www.geeksforgeeks.org
What are Bases? Definition, Examples, Types, Properties and Uses Bases Definition Class 10 A base is an ion or molecule that is able to accept a hydrogen ion from an acid. An acid is basically a molecule which can. In this post, we will discuss the. Acidic substances are usually identified by their sour taste. Usually, the acidic substances are identified with their sour taste. Bases release hydroxide ions (oh⁻), have a bitter. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. A base is a molecule or ion able to accept a hydrogen ion from an acid. An acid is basically a molecule which can. Dilution of acid and base: The concentration of hydrogen ion in an acid and hydroxide ion in. Bases Definition Class 10.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Bases Definition Class 10 In this post, we will discuss the. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. A base is an ion or molecule that is able to accept a hydrogen ion from an acid. Usually, the acidic substances are identified with their sour taste. A base is a molecule or. Bases Definition Class 10.
From www.expii.com
Strong and Weak Acids and Bases — Definition & Examples Expii Bases Definition Class 10 Both react to form salts and water in. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. A base is a molecule or ion able to accept a hydrogen ion from an acid. A base is an ion or molecule that is able to accept a hydrogen ion from an acid.. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. An acid is basically a molecule which can. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Usually, the acidic substances are identified with their sour taste.. Bases Definition Class 10.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1383826 Bases Definition Class 10 In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. A base is a molecule or ion able to accept a hydrogen ion from an acid. Both react to form salts and water in. The concentration of hydrogen ion in an acid and hydroxide ion in a base,. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Acidic substances are usually identified by their sour taste. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. An acid is basically a molecule which can. A base. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Dilution of acid and base: In this post, we will discuss the. An acid is basically a molecule which can. Usually, the acidic substances are identified with their sour taste. A base is a molecule or ion able to accept a. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. Both react to form salts and water in. While we might take them for granted in our everyday activities, it. Bases Definition Class 10.
From www.youtube.com
Acids, Bases and Salts Class 10 Full Chapter (Animation) Class 10 Bases Definition Class 10 Acidic substances are usually identified by their sour taste. While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. Both react to form salts and water in. Usually, the. Bases Definition Class 10.
From www.worksheetsplanet.com
What is a Base Definition of Base Bases Definition Class 10 In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. A base is an ion or molecule that is able to accept a hydrogen ion from. Bases Definition Class 10.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions Bases Definition Class 10 Dilution of acid and base: Acidic substances are usually identified by their sour taste. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide ions when dissolved in aqueous media. In class 10 chapter 2 notes, students learn about the difference between acids, bases and salts, their various properties, chemical reactions and. A base. Bases Definition Class 10.
From pediaa.com
Difference Between Strong and Weak Bases Definition, Properties Bases Definition Class 10 While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. Acidic substances are usually identified by their sour taste. Dilution of acid and base: Both react to form salts. Bases Definition Class 10.
From klagiicuo.blob.core.windows.net
Base Definition Chemistry Igcse at Carolyn Wright blog Bases Definition Class 10 Acidic substances are usually identified by their sour taste. In this post, we will discuss the. The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. Usually, the acidic substances are identified with their sour taste. In the field of chemistry, a ‘base’ can be defined as a substance that releases hydroxide. Bases Definition Class 10.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Acid Bases and Salts Bases Definition Class 10 The concentration of hydrogen ion in an acid and hydroxide ion in a base, per unit volume, shows. In this post, we will discuss the. Acidic substances are usually identified by their sour taste. Bases release hydroxide ions (oh⁻), have a bitter taste, a ph above 7, and turn red litmus paper blue. Dilution of acid and base: Both react. Bases Definition Class 10.