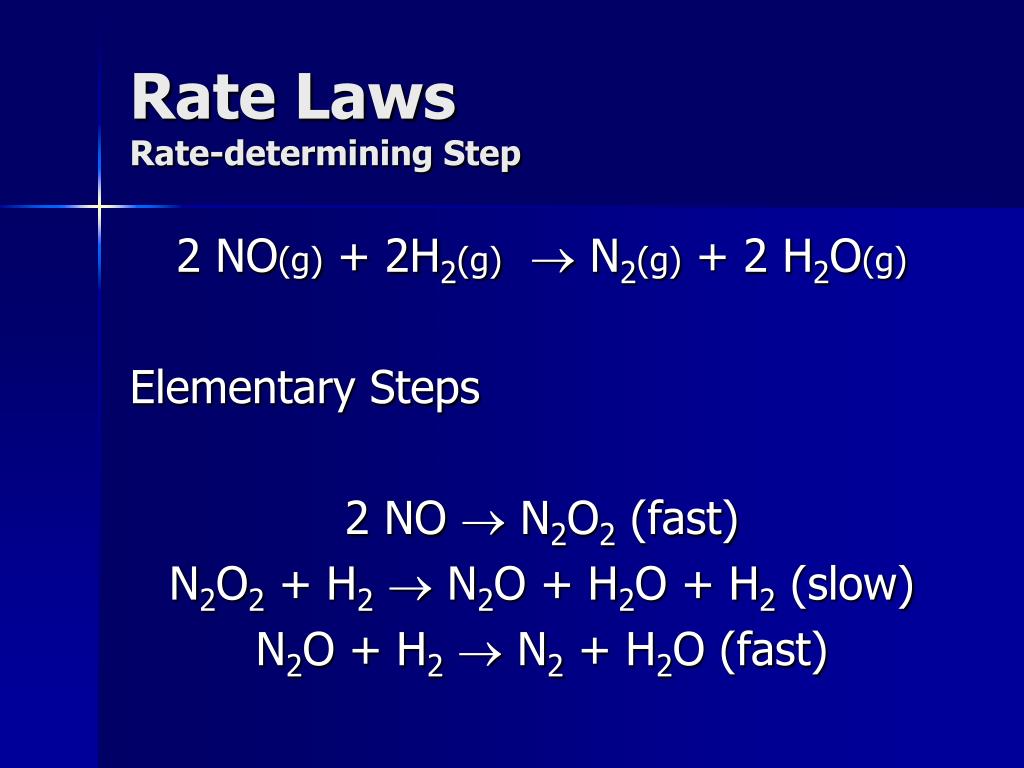
Are Solids And Liquids Included In Rate Law . Use rate and concentration data to identify reaction orders. Essentially, how do i resolve this apparent paradox: Use rate and concentration data to identify reaction orders and derive rate laws. Explain the form and function of a rate law. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. How fast one species appears or. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate laws to calculate reaction rates. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Are the algebraic equations that apply to a given reaction.
from www.slideserve.com
Use rate and concentration data to identify reaction orders and derive rate laws. Explain the form and function of a rate law. Explain the form and function of a rate law. How fast one species appears or. Use rate laws to calculate reaction rates. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Are the algebraic equations that apply to a given reaction. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Use rate and concentration data to identify reaction orders. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't.
PPT Rate Laws PowerPoint Presentation, free download ID6569323
Are Solids And Liquids Included In Rate Law When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Essentially, how do i resolve this apparent paradox: Explain the form and function of a rate law. Are the algebraic equations that apply to a given reaction. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. How fast one species appears or. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. How fast one species appears or. Use rate and concentration data to identify reaction orders. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. Use rate laws. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT Unit 9 and Equilibrium PowerPoint Presentation, free Are Solids And Liquids Included In Rate Law The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Explain the form and function of a rate law. Use rate and concentration data to identify reaction orders. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates.. Are Solids And Liquids Included In Rate Law.
From www.snexplores.org
Explainer What are the different states of matter? Are Solids And Liquids Included In Rate Law Are the algebraic equations that apply to a given reaction. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate and concentration data to identify reaction orders. Essentially, how do i resolve this apparent paradox:. Are Solids And Liquids Included In Rate Law.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Are Solids And Liquids Included In Rate Law The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Use rate and concentration data to identify reaction orders and derive rate laws. Explain the form and function of a rate law. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate and concentration data. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders and derive rate laws. Are the algebraic equations that apply to a given reaction. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate laws to calculate reaction rates. How fast one species appears or. Solids and liquids, as long as there is some available, are fully available to the. Are Solids And Liquids Included In Rate Law.
From saylordotorg.github.io
Solids and Liquids Are Solids And Liquids Included In Rate Law When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate and concentration data to identify reaction orders and derive rate laws. How fast one species appears or. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Use rate laws to calculate reaction rates. The. Are Solids And Liquids Included In Rate Law.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. How fast one species appears or. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. Solids and liquids, as long as there is some available, are fully available to the reaction. Are Solids And Liquids Included In Rate Law.
From libguides.bbc.qld.edu.au
States of Matter Junior School Brisbane Boys' College Library at Are Solids And Liquids Included In Rate Law The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Essentially, how do i resolve this apparent paradox: Are the algebraic equations that apply to a given reaction. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Use rate. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to. Are Solids And Liquids Included In Rate Law.
From dianaschemistrynotes.weebly.com
Chapter 2 CHEMISTRY NOTES Are Solids And Liquids Included In Rate Law The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Explain the form and function of a rate law. The rate. Are Solids And Liquids Included In Rate Law.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. Essentially, how do i resolve this apparent paradox: Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders and derive rate laws. Are the algebraic equations that apply to a given reaction. Use rate laws to. Are Solids And Liquids Included In Rate Law.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Are Solids And Liquids Included In Rate Law How fast one species appears or. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. The rate law is experimentally determined and can be used to predict. Are Solids And Liquids Included In Rate Law.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Are Solids And Liquids Included In Rate Law Explain the form and function of a rate law. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate laws to calculate reaction rates. When the system is. Are Solids And Liquids Included In Rate Law.
From www.youtube.com
Properties of Solids, Liquids and Gases YouTube Are Solids And Liquids Included In Rate Law Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders and derive rate laws. Explain the form and function of a rate law. The rate law is experimentally determined and can be used to predict the relationship between. Are Solids And Liquids Included In Rate Law.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Are Solids And Liquids Included In Rate Law Are the algebraic equations that apply to a given reaction. Explain the form and function of a rate law. How fast one species appears or. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Solids and liquids, as long as there is some available, are fully available to the reaction in. Are Solids And Liquids Included In Rate Law.
From www.numerade.com
SOLVED\( \mathrm{} \) \( \mathrm{} \) Are Solids And Liquids Included In Rate Law How fast one species appears or. Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Are the algebraic equations that apply to a given reaction. Use rate and concentration data to identify reaction orders. Essentially, how do i resolve this. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2224637 Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. Are the algebraic equations that apply to a given reaction. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law.. Are Solids And Liquids Included In Rate Law.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. How fast one species appears or. Essentially, how do i resolve this apparent paradox: When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Explain the form and. Are Solids And Liquids Included In Rate Law.
From sciencenotes.org
States of Matter Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID6569323 Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. How fast one species appears or. Explain the form and function of a rate law. Essentially, how do i resolve this apparent paradox: Explain the form and function of a rate law. Use rate and concentration data to identify reaction orders. The. Are Solids And Liquids Included In Rate Law.
From middleschoolscience.com
Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School Are Solids And Liquids Included In Rate Law Explain the form and function of a rate law. How fast one species appears or. Explain the form and function of a rate law. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. Use rate and concentration data. Are Solids And Liquids Included In Rate Law.
From slideplayer.com
Introduction to the Equilibrium Constant ppt download Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount. Are Solids And Liquids Included In Rate Law.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. Use rate laws to calculate reaction rates. How fast one species appears or. Essentially, how do i resolve this apparent paradox: Use rate and concentration data to identify reaction orders. Explain the form and function of a rate law. Use rate and concentration data to identify reaction orders and derive rate laws. Are. Are Solids And Liquids Included In Rate Law.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Are Solids And Liquids Included In Rate Law How fast one species appears or. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Use rate. Are Solids And Liquids Included In Rate Law.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Are Solids And Liquids Included In Rate Law When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Use rate and concentration data to identify reaction orders. Use rate and concentration data to identify reaction orders and derive rate laws. Use rate laws to calculate reaction rates. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Explain the form and function of. Are Solids And Liquids Included In Rate Law.
From www.britannica.com
phase Definition & Facts Britannica Are Solids And Liquids Included In Rate Law Essentially, how do i resolve this apparent paradox: Are the algebraic equations that apply to a given reaction. Use rate laws to calculate reaction rates. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Use rate laws to calculate reaction rates. Explain the form and. Are Solids And Liquids Included In Rate Law.
From 7thgradescicvjh.weebly.com
Matter Guide 7TH GRADE SCIENCE Are Solids And Liquids Included In Rate Law Explain the form and function of a rate law. How fast one species appears or. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. When the system is. Are Solids And Liquids Included In Rate Law.
From www.ase.org.uk
Solids, liquids and gases Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT Review Expressions of the thermodynamic equilibrium constant K Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Essentially, how do i resolve this apparent paradox: Use rate and concentration data to identify reaction orders. The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. The rate law. Are Solids And Liquids Included In Rate Law.
From www.makescienceeasy.com
Ultimate Revision Guide Make Science Easy Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders and derive rate laws. Explain the form and function of a rate law. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Essentially, how do i resolve this apparent. Are Solids And Liquids Included In Rate Law.
From www.youtube.com
Difference between solid liquid and gas Molecules of solid liquid and Are Solids And Liquids Included In Rate Law When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Essentially, how do i resolve this apparent paradox: Explain the form and function of a rate law. Explain the form and function of a rate law.. Are Solids And Liquids Included In Rate Law.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Are Solids And Liquids Included In Rate Law Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. The rate law is a mathematical relationship obtained by comparing reaction rates with reactant concentrations. Use rate laws to calculate reaction rates. Are the algebraic equations that apply to a given reaction. Use rate and. Are Solids And Liquids Included In Rate Law.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Are Solids And Liquids Included In Rate Law Use rate laws to calculate reaction rates. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate and concentration data to identify reaction orders and derive rate laws. How fast one species appears or. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't.. Are Solids And Liquids Included In Rate Law.
From www.primaryworks.co.uk
PowerPoint KS2 explanation on solids, liquids & gases KS2 primary Are Solids And Liquids Included In Rate Law Use rate and concentration data to identify reaction orders. Solids and liquids, as long as there is some available, are fully available to the reaction in a macroscopic amount that does not limit the. Use rate laws to calculate reaction rates. When the system is at equilibrium, removing $\ce{a(s)}$ doesn't. The rate law is a mathematical relationship obtained by comparing. Are Solids And Liquids Included In Rate Law.
From es.slideshare.net
Tang 03 equilibrium law keq 2 Are Solids And Liquids Included In Rate Law The rate law is experimentally determined and can be used to predict the relationship between the rate of a reaction and the concentrations of. Use rate and concentration data to identify reaction orders. Use rate laws to calculate reaction rates. Use rate and concentration data to identify reaction orders and derive rate laws. The rate law is a mathematical relationship. Are Solids And Liquids Included In Rate Law.