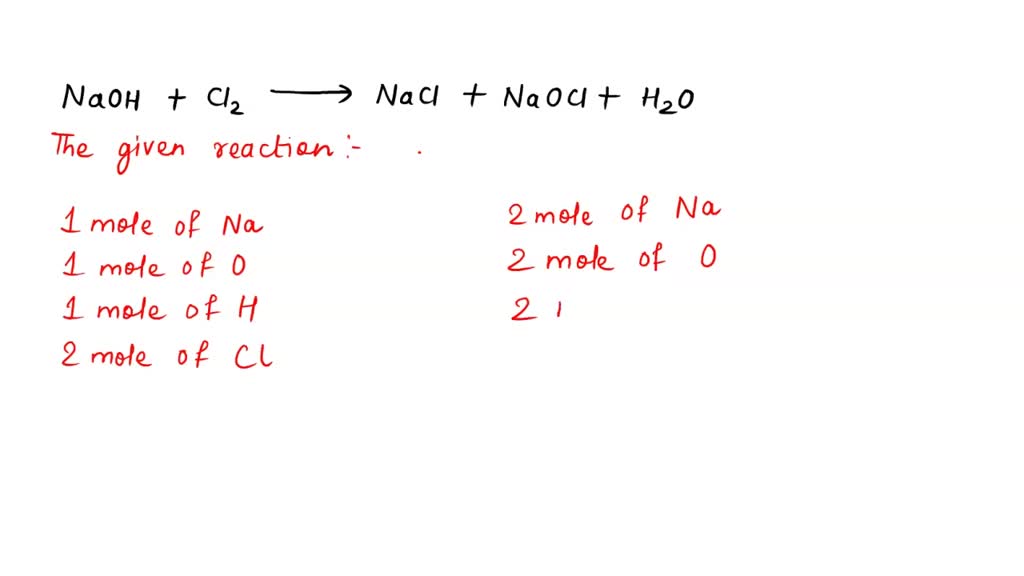
Chlorine Naoh Reaction . The reaction between chlorine and cold dilute sodium hydroxide solution is: Define disproportionation & describe the role of. Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Compare three types of electrolytic cells: The reaction that takes place is: 2 cl ̅ cl2 + 2 e ̅. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Naclo (sometimes written as naocl) is sodium chlorate(i). Use our revision notes to revise the reaction of chlorine with naoh.
from www.numerade.com
During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. The reaction between chlorine and cold dilute sodium hydroxide solution is: Compare three types of electrolytic cells: 2 h2o + 2 e ̅ h2 + 2 oh ̅. The reaction that takes place is: Naclo (sometimes written as naocl) is sodium chlorate(i). Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. Define disproportionation & describe the role of. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine).
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium
Chlorine Naoh Reaction 2 h2o + 2 e ̅ h2 + 2 oh ̅. Compare three types of electrolytic cells: During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Naclo (sometimes written as naocl) is sodium chlorate(i). 2 h2o + 2 e ̅ h2 + 2 oh ̅. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction between chlorine and cold dilute sodium hydroxide solution is: Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: The reaction that takes place is: 2 cl ̅ cl2 + 2 e ̅. Use our revision notes to revise the reaction of chlorine with naoh. Define disproportionation & describe the role of. Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Naoh Reaction Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. The reaction that takes place is: Define disproportionation & describe the role of. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq). Chlorine Naoh Reaction.
From www.chegg.com
Solved The reaction of benzene with chlorine(Cl2) in the Chlorine Naoh Reaction The reaction between chlorine and cold dilute sodium hydroxide solution is: 2 cl ̅ cl2 + 2 e ̅. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). 2 h2o + 2 e ̅ h2 + 2 oh ̅. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed. Chlorine Naoh Reaction.
From www.youtube.com
How to Balance NaOH + Cl2 = NaCl + NaClO + H2O (Dilute Sodium hydroxide Chlorine Naoh Reaction Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. Compare three types of electrolytic cells: 2 cl ̅ cl2 + 2 e ̅. The reaction that takes place is: During electrolysis, chlorine is formed at the. Chlorine Naoh Reaction.
From byjus.com
Chlorine reacts with hot and concentrated NaOH and produces compounds X Chlorine Naoh Reaction Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Define disproportionation & describe the role of. Use our revision notes to revise. Chlorine Naoh Reaction.
From byjus.com
In the free radical chlorination of methane, the chain initiation step Chlorine Naoh Reaction The reaction between chlorine and cold dilute sodium hydroxide solution is: 2 cl ̅ cl2 + 2 e ̅. Compare three types of electrolytic cells: 2 h2o + 2 e ̅ h2 + 2 oh ̅. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: The reaction that takes place is:. Chlorine Naoh Reaction.
From www.numerade.com
SOLVED Write the Sn1 reaction for tertbutyl bromide + NaOH, tert Chlorine Naoh Reaction Compare three types of electrolytic cells: Use our revision notes to revise the reaction of chlorine with naoh. 2 cl ̅ cl2 + 2 e ̅. The reaction that takes place is: 2 h2o + 2 e ̅ h2 + 2 oh ̅. The reaction between chlorine and cold dilute sodium hydroxide solution is: Define disproportionation & describe the role. Chlorine Naoh Reaction.
From askfilo.com
(p) When chlorine dissolves in water the following reaction occurs. NaOH+.. Chlorine Naoh Reaction Define disproportionation & describe the role of. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction that takes place is: During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. The reaction between chlorine and cold dilute sodium hydroxide solution is: Naclo (sometimes written. Chlorine Naoh Reaction.
From askfilo.com
Chlorine reacts with cold and dilute KOH and gives disproportionate react.. Chlorine Naoh Reaction 2 cl ̅ cl2 + 2 e ̅. Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. 2 h2o + 2 e ̅ h2 + 2 oh ̅. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Cl2 (aq) + 2naoh (aq). Chlorine Naoh Reaction.
From www.youtube.com
Cl_ 2 on reaction with hot & conc. NaOH gives two chlorine having Chlorine Naoh Reaction Naclo (sometimes written as naocl) is sodium chlorate(i). Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: 2 cl ̅ cl2 + 2 e ̅. 2 h2o + 2 e ̅ h2 + 2 oh ̅.. Chlorine Naoh Reaction.
From studylib.net
CHLORINE (Cl ) SODIUM HYDROXIDE (NaOH) 2 Chlorine Naoh Reaction 2 cl ̅ cl2 + 2 e ̅. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). 2 h2o + 2 e ̅ h2 + 2 oh ̅. Define disproportionation & describe the role of. Use our revision notes to revise the reaction of chlorine with naoh. Compare three types of electrolytic cells: Naclo. Chlorine Naoh Reaction.
From scienceinfo.com
Chloroform Preparation, Properties, Reactions, Uses, Chlorine Naoh Reaction The reaction that takes place is: Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Naclo (sometimes written as naocl) is sodium chlorate(i). During electrolysis, chlorine is formed at the anode and hydrogen. Chlorine Naoh Reaction.
From meetingtarget11.gitlab.io
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis Chlorine Naoh Reaction 2 cl ̅ cl2 + 2 e ̅. Define disproportionation & describe the role of. Naclo (sometimes written as naocl) is sodium chlorate(i). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction between chlorine. Chlorine Naoh Reaction.
From www.numerade.com
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium Chlorine Naoh Reaction 2 cl ̅ cl2 + 2 e ̅. Naclo (sometimes written as naocl) is sodium chlorate(i). Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction that takes place is: Use our revision notes to revise the reaction of chlorine with naoh. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq). Chlorine Naoh Reaction.
From www.youtube.com
Sodium and Chlorine Reaction YouTube Chlorine Naoh Reaction The reaction that takes place is: Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction between chlorine and cold dilute sodium hydroxide solution is: Define disproportionation & describe the role of. Compare three types. Chlorine Naoh Reaction.
From www.numerade.com
SOLVED Calcium hypochlorite, Ca(OCl)2, is a bleaching agent produced Chlorine Naoh Reaction Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: 2 cl ̅ cl2 + 2 e ̅. Compare three types of electrolytic cells: Naclo (sometimes written as naocl) is sodium chlorate(i). Use our revision notes to. Chlorine Naoh Reaction.
From www.toppr.com
How chlorine react with Cold and dilute NaoH? Chlorine Naoh Reaction Naclo (sometimes written as naocl) is sodium chlorate(i). Define disproportionation & describe the role of. Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Compare three types of electrolytic cells: 2 cl ̅. Chlorine Naoh Reaction.
From byjus.com
In electrophilic substitution reaction of chlorobenzene, the ortho/para Chlorine Naoh Reaction During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. 2 h2o + 2 e ̅ h2 + 2 oh ̅. The reaction that takes place is: Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. Compare three types of electrolytic cells: 2. Chlorine Naoh Reaction.
From www.meritnation.com
Chlorobenzene in the presence of NaOH at 623 K, 300 atm pressure gives Chlorine Naoh Reaction 2 h2o + 2 e ̅ h2 + 2 oh ̅. Naclo (sometimes written as naocl) is sodium chlorate(i). The reaction that takes place is: Define disproportionation & describe the role of. Use our revision notes to revise the reaction of chlorine with naoh. The reaction between chlorine and cold dilute sodium hydroxide solution is: Learn how sodium hydroxide, naoh,. Chlorine Naoh Reaction.
From studylib.net
CHLORINE (Cl ) SODIUM HYDROXIDE (NaOH) AND Chlorine Naoh Reaction During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Define disproportionation & describe the role of. 2 h2o + 2 e ̅ h2 + 2 oh ̅. 2 cl ̅ cl2 + 2. Chlorine Naoh Reaction.
From www.youtube.com
Reaction of Sodium with Chlorine (Na+Cl2) YouTube Chlorine Naoh Reaction The reaction that takes place is: 2 cl ̅ cl2 + 2 e ̅. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Use our revision notes to revise the reaction of chlorine with naoh. The reaction between chlorine and. Chlorine Naoh Reaction.
From www.numerade.com
SOLVEDWhich chlorine is first substituted on reaction with NaOH Chlorine Naoh Reaction The reaction that takes place is: Compare three types of electrolytic cells: Use our revision notes to revise the reaction of chlorine with naoh. 2 cl ̅ cl2 + 2 e ̅. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction. Chlorine Naoh Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for NH4Cl + NaOH = NaCl + H2O + NH3 Chlorine Naoh Reaction Use our revision notes to revise the reaction of chlorine with naoh. Naclo (sometimes written as naocl) is sodium chlorate(i). Compare three types of electrolytic cells: Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at. Chlorine Naoh Reaction.
From www.toppr.com
When chlorine reacts with cold and dilute solution of sodium hydroxide Chlorine Naoh Reaction During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Define disproportionation & describe the role of. Use our revision notes to revise the reaction of chlorine with naoh. The reaction between chlorine and cold dilute sodium. Chlorine Naoh Reaction.
From www.numerade.com
SOLVED Below is a graph of the concentration of chlorine dioxide Chlorine Naoh Reaction Compare three types of electrolytic cells: 2 cl ̅ cl2 + 2 e ̅. The reaction between chlorine and cold dilute sodium hydroxide solution is: Use our revision notes to revise the reaction of chlorine with naoh. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: During electrolysis, chlorine is formed. Chlorine Naoh Reaction.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry Chlorine Naoh Reaction 2 cl ̅ cl2 + 2 e ̅. The reaction that takes place is: Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Naclo (sometimes written as naocl) is sodium chlorate(i). Compare three types of electrolytic cells: Learn how sodium. Chlorine Naoh Reaction.
From www.mdpi.com
A Review of Traditional and Emerging Residual Chlorine Quenchers on Chlorine Naoh Reaction Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Naclo (sometimes written as naocl) is sodium chlorate(i). Use our revision notes to revise the reaction of chlorine with naoh. During electrolysis, chlorine is formed at the. Chlorine Naoh Reaction.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Naoh Reaction During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Compare three types of electrolytic cells: Define disproportionation & describe the role of. 2 h2o + 2 e ̅ h2 + 2 oh ̅. The reaction between chlorine and cold dilute sodium hydroxide solution is: Use our revision notes to revise the. Chlorine Naoh Reaction.
From www.toppr.com
How is Chlorine prepared by electrolyti method? Explain its reaction Chlorine Naoh Reaction Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). 2 cl ̅ cl2 + 2 e ̅. Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: 2 h2o + 2 e ̅ h2 + 2 oh ̅. Naclo (sometimes written as naocl) is sodium chlorate(i).. Chlorine Naoh Reaction.
From askfilo.com
Chlorine react with hot and conc. NaOH to form sodium chloride and sodium.. Chlorine Naoh Reaction The reaction between chlorine and cold dilute sodium hydroxide solution is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Use our revision notes to revise the reaction of chlorine with naoh. Compare three types of electrolytic cells: 2 cl ̅ cl2 + 2 e ̅. Naclo (sometimes written as naocl) is sodium chlorate(i).. Chlorine Naoh Reaction.
From www.numerade.com
SOLVED Given the following chemical equation for the reaction of Chlorine Naoh Reaction Define disproportionation & describe the role of. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Use our revision notes to revise the reaction of chlorine with naoh. Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide. Chlorine Naoh Reaction.
From byjus.com
when propene reacts with CL2 in light gives A . A reacts with chlorine Chlorine Naoh Reaction During electrolysis, chlorine is formed at the anode and hydrogen and hydroxide ions are formed at the cathode. Use our revision notes to revise the reaction of chlorine with naoh. Compare three types of electrolytic cells: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). The reaction that takes place is: Naclo (sometimes written. Chlorine Naoh Reaction.
From www.vrogue.co
What Happen When Chlorine Gas React With Naoh Solutio vrogue.co Chlorine Naoh Reaction 2 h2o + 2 e ̅ h2 + 2 oh ̅. Naclo (sometimes written as naocl) is sodium chlorate(i). Compare three types of electrolytic cells: Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Use our revision notes to revise the reaction of chlorine with naoh. 2 cl ̅ cl2 +. Chlorine Naoh Reaction.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Chlorine Naoh Reaction The reaction that takes place is: Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. The reaction between. Chlorine Naoh Reaction.
From www.youtube.com
Reaction between Sodium hydroxide and nickel(ii)chloride YouTube Chlorine Naoh Reaction Chlorine gas reacts with sodium hydroxide to form a salt of sodium chloride, sodium chlorate (i) and water. Define disproportionation & describe the role of. 2 h2o + 2 e ̅ h2 + 2 oh ̅. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Use our revision notes to revise the reaction of. Chlorine Naoh Reaction.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Chlorine Naoh Reaction The reaction that takes place is: 2 cl ̅ cl2 + 2 e ̅. Define disproportionation & describe the role of. Learn how sodium hydroxide, naoh, is produced by electrolysis of concentrated sodium chloride solutions (brine). Cl2 (aq) + 2naoh (aq) → nacl (aq) + naclo (aq) + h2o (l) the ionic equation is: The reaction between chlorine and cold. Chlorine Naoh Reaction.