What Is E2B Reporting . this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. postmarketing safety reporting. what is e2b r2? How do i prepare and submit an e2b r2 adverse event report file? this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. e2b (r3) individual case safety report (icsr) specification and related files. How are submitted xml files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. The ich e2b ewg released an e2b guideline for.
from docs.oracle.com
postmarketing safety reporting. How are submitted xml files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. The ich e2b ewg released an e2b guideline for. what is e2b r2? Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. e2b (r3) individual case safety report (icsr) specification and related files. How do i prepare and submit an e2b r2 adverse event report file? this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual.
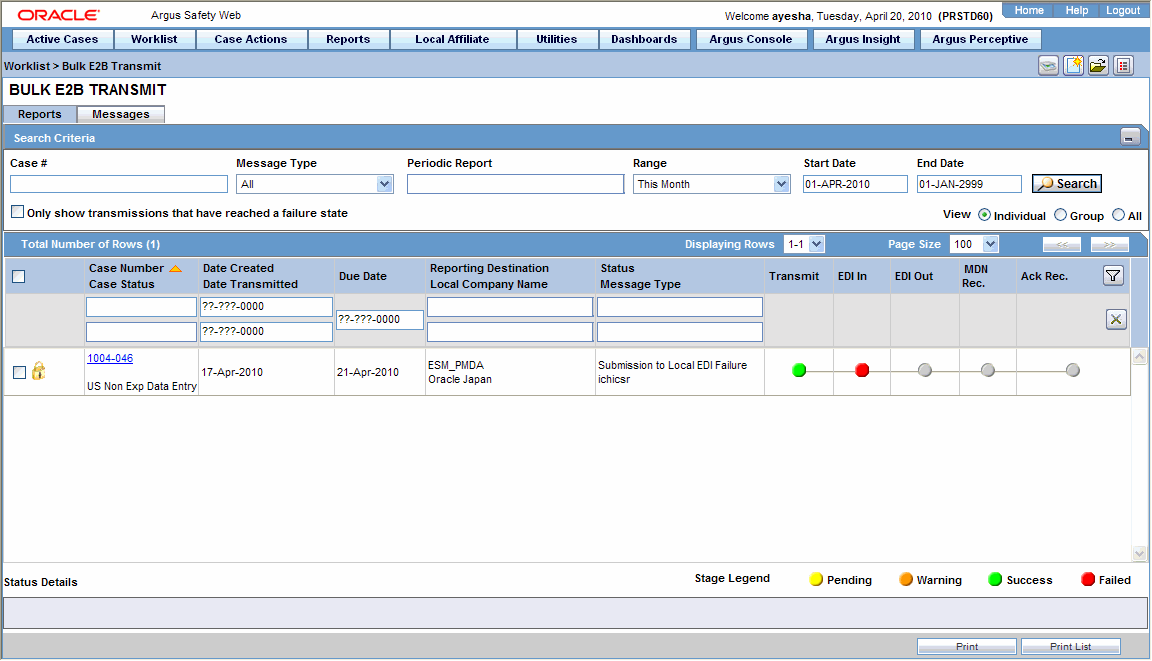
Transmitting and Monitoring E2B Reports
What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. postmarketing safety reporting. The ich e2b ewg released an e2b guideline for. what is e2b r2? How are submitted xml files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How do i prepare and submit an e2b r2 adverse event report file? this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. e2b (r3) individual case safety report (icsr) specification and related files. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B Reporting this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. The ich e2b ewg released an e2b guideline for. what is e2b r2? How are submitted xml files. e2b (r3) individual case safety report (icsr) specification and related files. Marketing authorisation holders and sponsors of clinical trials must. What Is E2B Reporting.
From dokumen.tips
(PDF) What's E2B Viewer? DOKUMEN.TIPS What Is E2B Reporting How are submitted xml files. How do i prepare and submit an e2b r2 adverse event report file? Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. postmarketing safety reporting. what is e2b r2? this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich. What Is E2B Reporting.
From docs.oracle.com
Using the E2B Viewer What Is E2B Reporting How are submitted xml files. The ich e2b ewg released an e2b guideline for. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. e2b (r3) individual case safety report (icsr) specification and related files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. what is e2b r2? . What Is E2B Reporting.
From docs.oracle.com
System Configuration What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. The ich e2b ewg released an e2b guideline for. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. e2b (r3) individual case safety report (icsr) specification and related files. How are submitted xml files. this information paper explains the. What Is E2B Reporting.
From dokumen.tips
(PDF) What is E2B Marketing? DOKUMEN.TIPS What Is E2B Reporting what is e2b r2? How do i prepare and submit an e2b r2 adverse event report file? In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. e2b (r3) individual case safety report (icsr) specification and related files. How are submitted xml files. this information paper explains the short history of e2b,. What Is E2B Reporting.
From docs.oracle.com
Reports Bulk Reporting, E2B Pending, and Processed E2B Reports What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. e2b (r3) individual case safety report (icsr) specification and related files. How do i prepare and submit an e2b r2 adverse event. What Is E2B Reporting.
From docs.oracle.com
Extending through E2B PMDA Profile What Is E2B Reporting How are submitted xml files. what is e2b r2? Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. e2b (r3) individual case safety report (icsr) specification and related files. postmarketing safety reporting. this information paper explains the. What Is E2B Reporting.
From docs.oracle.com
System Configuration What Is E2B Reporting e2b (r3) individual case safety report (icsr) specification and related files. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. what is e2b r2? The ich e2b ewg released an e2b guideline for. How. What Is E2B Reporting.
From docs.oracle.com
Transmitting and Monitoring E2B Reports What Is E2B Reporting this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. postmarketing safety. What Is E2B Reporting.
From who-umc.org
VigiFlow eReporting for Industry UMC What Is E2B Reporting How are submitted xml files. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission. What Is E2B Reporting.
From ceqzjvlt.blob.core.windows.net
What Is An E2B File at David Rogers blog What Is E2B Reporting what is e2b r2? How do i prepare and submit an e2b r2 adverse event report file? postmarketing safety reporting. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. e2b (r3) individual case safety report (icsr) specification and. What Is E2B Reporting.
From docs.oracle.com
Transmitting and Monitoring E2B Reports What Is E2B Reporting In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. what is e2b r2? postmarketing safety reporting. The ich e2b. What Is E2B Reporting.
From ceqzjvlt.blob.core.windows.net
What Is An E2B File at David Rogers blog What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. How do i prepare and submit an e2b r2 adverse event report file? this document provides guidance on the implementation of the standard adopted. What Is E2B Reporting.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. e2b (r3) individual case safety report (icsr) specification and related files. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. postmarketing safety reporting. what is e2b r2? this document provides. What Is E2B Reporting.
From docs.oracle.com
Configuring Documentum What Is E2B Reporting How are submitted xml files. How do i prepare and submit an e2b r2 adverse event report file? postmarketing safety reporting. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. e2b (r3) individual case. What Is E2B Reporting.
From docs.oracle.com
Importing E2B Reports What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. postmarketing safety reporting. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of. What Is E2B Reporting.
From www.youtube.com
Pharmacovigilance guideline E2B(R3) PART2 YouTube What Is E2B Reporting In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. The ich e2b ewg released an e2b guideline for. e2b (r3) individual case safety report (icsr) specification and related files. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. How do i prepare and submit an e2b r2 adverse event. What Is E2B Reporting.
From www.datacreds.com
Data Integrity Drive E2B XMLs and the Assurance of Accurate Reporting What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. The ich e2b ewg released an e2b guideline for. How do i prepare and submit an e2b r2 adverse event report file? In preparation for. What Is E2B Reporting.
From ceqzjvlt.blob.core.windows.net
What Is An E2B File at David Rogers blog What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. postmarketing safety reporting. How do i prepare and submit an e2b r2 adverse event report file? e2b (r3) individual case safety report (icsr) specification and related files. what is e2b r2? In preparation for the receipt of postmarketing safety reports in the e2b (r3). What Is E2B Reporting.
From hiropharmaconsulting.com
FDA E2B(R3)/ FARESII Seminar Report HiroPharmaConsulting What Is E2B Reporting The ich e2b ewg released an e2b guideline for. what is e2b r2? How are submitted xml files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. postmarketing safety reporting.. What Is E2B Reporting.
From docs.oracle.com
E2B Extensions (Applicable for R2 only) What Is E2B Reporting In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. postmarketing safety reporting. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. How do i prepare and submit an e2b r2 adverse event report file? what is e2b r2? e2b (r3) individual case safety report (icsr) specification and. What Is E2B Reporting.
From allaboutpharmacovigilance.org
Understanding E2B Pharmacovigilance What Is E2B Reporting The ich e2b ewg released an e2b guideline for. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. e2b (r3) individual case safety report (icsr) specification and related files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How do. What Is E2B Reporting.
From support.ezeep.com
ezeep blue tracking & reporting ezeep Blue What Is E2B Reporting e2b (r3) individual case safety report (icsr) specification and related files. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. what is e2b r2? How do i prepare and submit an e2b r2 adverse event report file? postmarketing safety reporting. How are submitted xml files. this information paper explains the short history. What Is E2B Reporting.
From docs.oracle.com
Using the E2B Viewer What Is E2B Reporting How are submitted xml files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. postmarketing safety reporting. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. this document provides guidance on the implementation of the standard adopted by the. What Is E2B Reporting.
From www.datacreds.com
Reporting Reimagined E2B XMLs Setting New Standards in Pharmacovigilance What Is E2B Reporting e2b (r3) individual case safety report (icsr) specification and related files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. what is e2b r2? this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. Marketing authorisation holders and sponsors of clinical trials. What Is E2B Reporting.
From www.youtube.com
E2B Podcast Bringing Clarity To SMOG Reporting YouTube What Is E2B Reporting e2b (r3) individual case safety report (icsr) specification and related files. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How are submitted xml files. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. Marketing authorisation holders and sponsors of. What Is E2B Reporting.
From docs.oracle.com
Transmitting and Monitoring E2B Reports What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How are submitted xml files. postmarketing safety reporting. The ich e2b ewg released an e2b guideline for. what is e2b r2?. What Is E2B Reporting.
From news.evidentiq.com
E2B Safety Notifications Get a free demo What Is E2B Reporting this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. e2b (r3) individual case safety report (icsr) specification and related files. The ich e2b ewg released an e2b guideline for. what is e2b r2? postmarketing safety reporting. How do i prepare and submit an e2b. What Is E2B Reporting.
From docs.oracle.com
Transmitting and Monitoring E2B Reports What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. what is e2b r2? How do i prepare and submit an e2b r2 adverse event report file? this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. e2b (r3) individual case safety report (icsr) specification. What Is E2B Reporting.
From e2btek.com
e2b teknologies BUSINESS INTELLIGENCE & REPORTING What Is E2B Reporting How do i prepare and submit an e2b r2 adverse event report file? this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. How are submitted xml files. The ich e2b ewg released an e2b guideline for. In preparation for the receipt of postmarketing safety reports in the. What Is E2B Reporting.
From docs.oracle.com
Validate ICSR Reports What Is E2B Reporting Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. How do i prepare and submit an e2b r2 adverse event report file? postmarketing safety reporting. How are submitted xml files. what is e2b r2? The ich e2b ewg released an e2b guideline for. In preparation for the receipt of postmarketing safety reports in the. What Is E2B Reporting.
From docs.oracle.com
Report Features What Is E2B Reporting what is e2b r2? In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. postmarketing safety reporting. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected. this information paper explains the short history of e2b, relationship to ich m5 and iso idmp standard, and ich position on. . What Is E2B Reporting.
From www.slideshare.net
E2B(R2) vs E2B(R3) ICSR ELEMENTS What Is E2B Reporting postmarketing safety reporting. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. How do i prepare and submit an e2b r2 adverse event report file? The ich e2b ewg released an e2b guideline for. this information paper explains the short history of e2b, relationship to ich m5. What Is E2B Reporting.
From www.datacreds.com
Simplified Reporting E2B XMLs' Mission in Streamlining Adverse Event What Is E2B Reporting In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How are submitted xml files. this document provides guidance on the implementation of the standard adopted by the ich for electronic transmission of individual. How do i prepare and submit an e2b r2 adverse event report file? e2b (r3) individual case safety report. What Is E2B Reporting.
From slideplayer.com
Magnus Wallberg Dar Es Salaam, Tanzania November 25 th, 2009 VigiFlow What Is E2B Reporting In preparation for the receipt of postmarketing safety reports in the e2b (r3) format, fda. How do i prepare and submit an e2b r2 adverse event report file? e2b (r3) individual case safety report (icsr) specification and related files. postmarketing safety reporting. The ich e2b ewg released an e2b guideline for. How are submitted xml files. Marketing authorisation. What Is E2B Reporting.