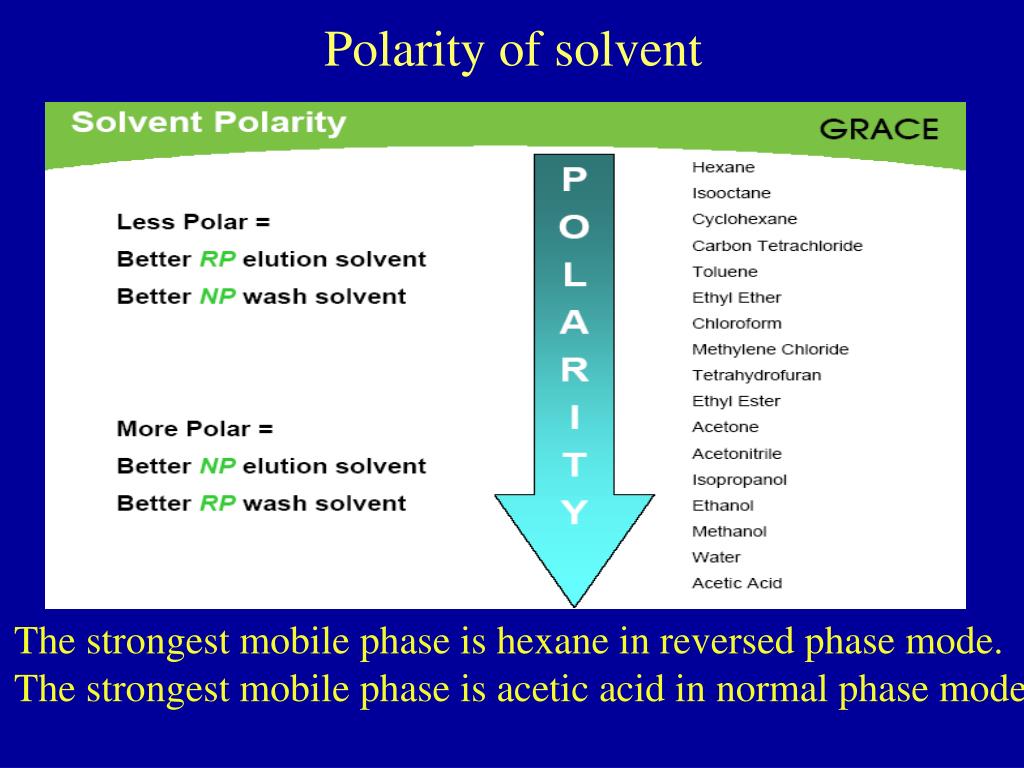
Normal Phase Hplc Solvent Strength . Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. The solvent strength ε of the mobile phase, eq.
from www.slideserve.com
The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar.
PPT HPLC PowerPoint Presentation, free download ID4619366
Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq.
From www.researchgate.net
Comparison of ethanol and acetonitrile in the reversedphase HPLC Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From bceweb.org
Hplc Solvent Strength Chart A Visual Reference of Charts Chart Master Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) PowerPoint Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From ymc.de
Normal Phase (NP) HPLC Columns YMC Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC of the first intermediates of toluene and oxylene Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC chromatograms showing (A) separation of the major Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normal phase, gradientHPLC analysis of FCH and [ 14 C]CH (A); PFCH Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT Chemistry 4631 PowerPoint Presentation, free download ID5429136 Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From mavink.com
Hplc Solvent Strength Chart Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC chromatograms of the reaction product obtained from Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normal phase HPLC separation of 2AB derivatized Nlinked glycans from Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT What Is HPLC? PowerPoint Presentation, free download ID4713396 Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normal phase HPLC analysis of 2ABlabeled Nlinked glycans isolated Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT Reversed Phase HPLC Mechanisms PowerPoint Presentation ID260343 Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT Chemistry 4631 PowerPoint Presentation, free download ID5429136 Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. Normal Phase Hplc Solvent Strength.
From bceweb.org
Hplc Solvent Strength Chart A Visual Reference of Charts Chart Master Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Solvent polarity and eluotropic strength (ɛ°) Download Scientific Diagram Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC analysis of a fraction from the PRLcatalyzed Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
A, straight phase HPLC analysis of A 4 /J 4NPs generated from the Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Relationship between retention factor and solvent strength measured for Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT Chemistry 4631 PowerPoint Presentation, free download ID5429136 Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. Normal Phase Hplc Solvent Strength.
From slideplayer.com
High performance liquid chromatograph ppt download Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From bceweb.org
Hplc Solvent Strength Chart A Visual Reference of Charts Chart Master Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT Chemistry 4631 PowerPoint Presentation, free download ID5429136 Normal Phase Hplc Solvent Strength This is a measure of the interaction of. The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT NORMALPHASE CHROMATOGRAPHY PowerPoint Presentation, free Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC of 8 tocochromanols in different samples. (a) Mixture Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC chromatogram of mixed PPL standards (PE Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From searchforhappiness.eu
ELUOTROPIC SERIES PDF Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From mavink.com
Normal Phase Hplc Diagram Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From slidetodoc.com
High Performance Liquid Chromatography HPLC originally refered to Normal Phase Hplc Solvent Strength This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Normal Phase Hplc Solvent Strength.
From hxeesuejy.blob.core.windows.net
What Is Normal Phase Hplc at Nichole Croom blog Normal Phase Hplc Solvent Strength The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. Normal Phase Hplc Solvent Strength.
From bceweb.org
Hplc Solvent Strength Chart A Visual Reference of Charts Chart Master Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From chempedia.info
NormalPhase Solvents Big Chemical Encyclopedia Normal Phase Hplc Solvent Strength Biggest problem with normal phase is the effect that water has on the activity of polar. Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). The solvent strength ε of the mobile phase, eq. This is a measure of the interaction of. Normal Phase Hplc Solvent Strength.
From www.researchgate.net
Normalphase HPLC chromatograms (monitored at 460 nm) of lycopene Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). This is a measure of the interaction of. Biggest problem with normal phase is the effect that water has on the activity of polar. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.
From www.slideserve.com
PPT HPLC PowerPoint Presentation, free download ID4619366 Normal Phase Hplc Solvent Strength Solvent strength (polarity) the strengths of various solvents are determined empirically and are listed in a eluotropic series (see solvents ). Biggest problem with normal phase is the effect that water has on the activity of polar. This is a measure of the interaction of. The solvent strength ε of the mobile phase, eq. Normal Phase Hplc Solvent Strength.