Fda Medical Device Verification And Validation . Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Effectiveness and savings into your medical device. The information on this page is current as of mar 22, 2024. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use.
from studylib.net
Effectiveness and savings into your medical device. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of mar 22, 2024. Additional copies are available from:
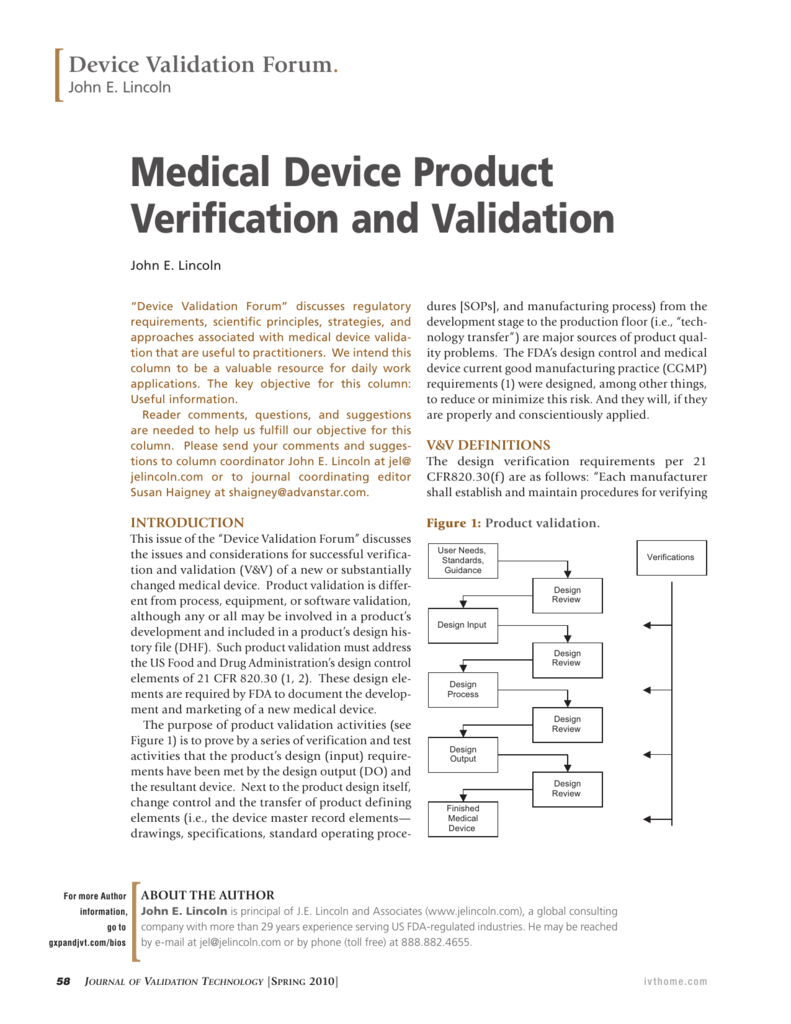
Medical Device Product Verification and Validation
Fda Medical Device Verification And Validation Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of mar 22, 2024. Effectiveness and savings into your medical device.
From www.youtube.com
Difference between Verification and Validation ISO 9001 Definitions Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Effectiveness and savings into your medical device. The information on this page is current as of mar 22, 2024. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit. Fda Medical Device Verification And Validation.
From qaconsultinginc.com
Design Verification vs. Design Validation What’s The Difference? QA Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. The information on this page is current as of mar 22, 2024. Additional copies. Fda Medical Device Verification And Validation.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Fda Medical Device Verification And Validation Additional copies are available from: The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From studylib.net
Medical Device Product Verification and Validation Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Additional copies. Fda Medical Device Verification And Validation.
From www.getreskilled.com
What's a Pharmaceutical Equipment Validation Protocol & Why is it Crucial? Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit. Fda Medical Device Verification And Validation.
From www.emergobyul.com
Analysis of design validation and verification for medical device human Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of. Fda Medical Device Verification And Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Fda Medical Device Verification And Validation Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. The information on this page is current as of. Fda Medical Device Verification And Validation.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of mar 22, 2024. Additional copies. Fda Medical Device Verification And Validation.
From www.presentationeze.com
Medical Device FDA Medical Device FDA GMP Audit GMPPresentationEZE Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of. Fda Medical Device Verification And Validation.
From www.aplyon.com
Design Verification and Validation Procedure Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: The information on this page is current as of. Fda Medical Device Verification And Validation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of mar 22, 2024. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From www.aligned.ch
Reaping the Benefits of Agile Testing in Medical Device Development Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Effectiveness and savings into your medical device. The information on this page is current as of. Fda Medical Device Verification And Validation.
From rs-ness.com
Process Validation Pharma vs. Medical Device RS NESS Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Additional copies are available from: Effectiveness and savings into. Fda Medical Device Verification And Validation.
From www.padtinc.com
FDA Opening to Simulation Supported Verification and Validation for Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of. Fda Medical Device Verification And Validation.
From academy.qualitymeddev.com
Medical Device Software Verification & Validation QualityMedDev Academy Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Additional copies. Fda Medical Device Verification And Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Effectiveness and savings into your medical device. Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that. Fda Medical Device Verification And Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Fda Medical Device Verification And Validation Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. The information on this page is current as of. Fda Medical Device Verification And Validation.
From www.qualitymeddev.com
Design Verification vs Design Validation What are The Differences Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Additional copies are available from: The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit. Fda Medical Device Verification And Validation.
From www.greenlight.guru
Beginner's Guide to Design Verification & Design Validation for Medical Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Additional copies. Fda Medical Device Verification And Validation.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Additional copies are available from: Effectiveness and savings into your medical device. The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that. Fda Medical Device Verification And Validation.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Additional copies. Fda Medical Device Verification And Validation.
From www.scribd.com
Medical Device Design Validation SOP Verification And Validation Fda Medical Device Verification And Validation Effectiveness and savings into your medical device. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: The information on this page is current as of. Fda Medical Device Verification And Validation.
From www.presentationeze.com
FDA Validation Requirements for Medical Devices PresentationEZE Fda Medical Device Verification And Validation Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of. Fda Medical Device Verification And Validation.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Fda Medical Device Verification And Validation Additional copies are available from: The information on this page is current as of mar 22, 2024. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that. Fda Medical Device Verification And Validation.
From www.presentationeze.com
Medical Device Process ValidationPresentationEZE Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of mar 22, 2024. Effectiveness and savings into your medical device. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit. Fda Medical Device Verification And Validation.
From bluefruit.co.uk
V&V Testing Signs you have a verification & validation issue Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: The information on this page is current as of mar 22, 2024. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From www.qualitymeddev.com
Process Validation for Medical Devices Overview of FDA Requirements Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From www.researchandmarkets.com
Understanding FDA Design Verification and Validation Requirements for Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of mar 22, 2024. Effectiveness and savings into. Fda Medical Device Verification And Validation.
From www.greenlight.guru
What Device Makers Need To Know About Design Verification and Validation Fda Medical Device Verification And Validation The information on this page is current as of mar 22, 2024. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that. Fda Medical Device Verification And Validation.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Verification And Validation Plan Template prntbl Fda Medical Device Verification And Validation Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Additional copies are available from: Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. The information on this page is current as of. Fda Medical Device Verification And Validation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Effectiveness and savings into your medical device. Additional copies are available from: Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. The information on this page is current as of. Fda Medical Device Verification And Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Fda Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Tions 21 cfr 820.3 (z)(1)process validationmeans establishing by objective evidence that a process consistently. Effectiveness and savings into your medical device. Additional copies are available from: The information on this page is current as of. Fda Medical Device Verification And Validation.