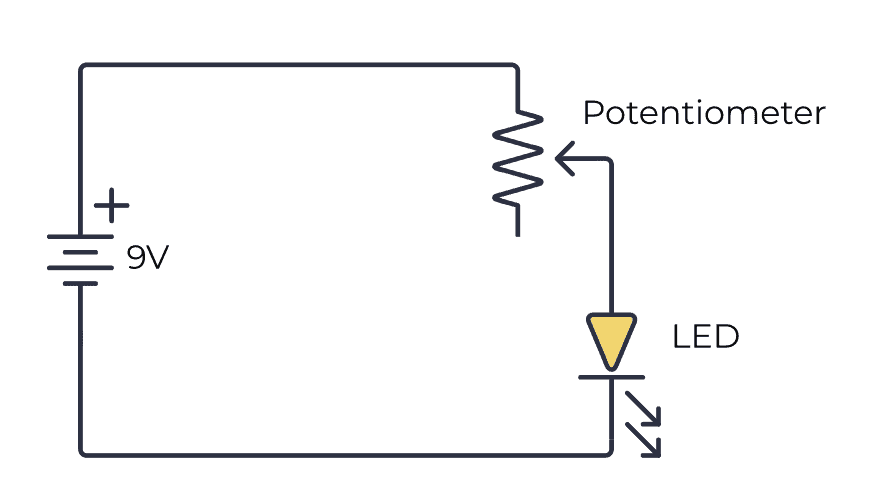
Potentiometer Reaction Definition . Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Because no current—or only a negligible current—flows through the. In potentiometry we measure the potential of an electrochemical cell under static conditions. The definition, the principle involved, curves, examples, and Find out how to use. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration.
from mylescartner.blogspot.com
Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. In potentiometry we measure the potential of an electrochemical cell under static conditions. The definition, the principle involved, curves, examples, and Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Because no current—or only a negligible current—flows through the. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Find out how to use.
18+ Potentiometer Pinout Diagram MylesCartner
Potentiometer Reaction Definition Find out how to use. Because no current—or only a negligible current—flows through the. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. The definition, the principle involved, curves, examples, and Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Find out how to use. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed.
From blog.pishop.co.za
Potentiometer Definition, Types and Working Principle Blog Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers. Potentiometer Reaction Definition.
From electropeak.com
How a Potentiometer Works And How to Use with Arduino [Full Guide] Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Learn how to use potentiometry to measure the potential of. Potentiometer Reaction Definition.
From www.doeeet.com
Basic Principles of Potentiometers/Variable Resistors Potentiometer Reaction Definition Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Find out how to use. In potentiometry we measure the potential of an electrochemical cell under static conditions. The definition, the principle involved,. Potentiometer Reaction Definition.
From www.youtube.com
Potentiometer Explained YouTube Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions. Potentiometer Reaction Definition.
From www.build-electronic-circuits.com
The Potentiometer Pinout, Wiring, and How It Works Potentiometer Reaction Definition Because no current—or only a negligible current—flows through the. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Learn about potentiometry, a classical analytical technique. Potentiometer Reaction Definition.
From electropeak.com
How a Potentiometer Works And How to Use with Arduino [Full Guide] Potentiometer Reaction Definition Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. In potentiometry we measure the potential of an electrochemical cell under static conditions. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Because no current—or only a negligible current—flows through the. Potentiometric. Potentiometer Reaction Definition.
From www.studocu.com
Potentiometer Definition, Types, And Working Principle Potentiometer Potentiometer Reaction Definition The definition, the principle involved, curves, examples, and Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Because no current—or only a negligible current—flows through the. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn about potentiometry,. Potentiometer Reaction Definition.
From fixpartandrea.z19.web.core.windows.net
Potentiometer Circuit Diagram Definition Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The definition, the principle involved, curves, examples, and Learn how to use potentiometry to measure the. Potentiometer Reaction Definition.
From www.theengineeringknowledge.com
What is a Potentiometer? Types, Uses, Working Principle & Applications Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Find out how to use. The definition, the principle involved, curves, examples, and Potentiometry is an electrochemical method that measures the electrical potential. Potentiometer Reaction Definition.
From mylescartner.blogspot.com
18+ Potentiometer Pinout Diagram MylesCartner Potentiometer Reaction Definition The definition, the principle involved, curves, examples, and Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration. Potentiometer Reaction Definition.
From www.linquip.com
What is Potentiometer? Diagram, Symbols, Characteristics Linquip Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. The definition, the principle involved, curves, examples, and Because no current—or only a negligible current—flows through the. Learn how to. Potentiometer Reaction Definition.
From testbook.com
Principle of Potentiometer Definition, Structure, Working, Uses Potentiometer Reaction Definition The definition, the principle involved, curves, examples, and Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Because no current—or only a negligible current—flows through the. In potentiometry we. Potentiometer Reaction Definition.
From www.aiophotoz.com
What Is A Potentiometer Definition Construction Working Principle Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the. Potentiometer Reaction Definition.
From epci.eu
Potentiometers Basic Principles Potentiometer Reaction Definition Because no current—or only a negligible current—flows through the. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. The definition, the principle involved, curves, examples, and Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn about potentiometry,. Potentiometer Reaction Definition.
From www.scribd.com
Potentiometer Definition, Types, and Working Principle PDF Potentiometer Reaction Definition Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Find out how to use. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Learn how to use potentiometry to measure the potential of an. Potentiometer Reaction Definition.
From www.youtube.com
All About Potentiometer, Potentiometer Connection, Working, Circuit Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Find out how to use. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using. Potentiometer Reaction Definition.
From medium.com
Comprehensive Guide to Potentiometers by Jotrinelectronic Medium Potentiometer Reaction Definition Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Because no current—or only a negligible current—flows through the. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Learn about potentiometry, a classical analytical technique based on the measurement. Potentiometer Reaction Definition.
From eduinput.com
Potentiometer Determination of Unknown emf Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible current—flows through the. The definition, the principle involved, curves, examples, and Potentiometric titration can be used to determine the concentration of. Potentiometer Reaction Definition.
From wiringdiagram.2bitboer.com
Wiring Diagram Two Potentiometers In Series Wiring Diagram Potentiometer Reaction Definition Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. The definition, the principle involved, curves, examples, and Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn about potentiometry, a classical analytical technique based on the measurement of. Potentiometer Reaction Definition.
From electropeak.com
How a Potentiometer Works And How to Use with Arduino [Full Guide] Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration can be used to determine. Potentiometer Reaction Definition.
From www.etechnog.com
[Proper] Potentiometer Connection and Circuit Diagram ETechnoG Potentiometer Reaction Definition Because no current—or only a negligible current—flows through the. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. The definition, the principle involved, curves, examples, and Potentiometric titration can be used to. Potentiometer Reaction Definition.
From www.basicsofelectricalengineering.com
Basics of Potentiometer Basics of Electrical Engineering Potentiometer Reaction Definition Find out how to use. In potentiometry we measure the potential of an electrochemical cell under static conditions. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to. Potentiometer Reaction Definition.
From study.com
Potentiometer Definition, Types & Examples Lesson Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible current—flows through the. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to. Potentiometer Reaction Definition.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Potentiometric titration can. Potentiometer Reaction Definition.
From www.youtube.com
What is a Potentiometer and how does it Work YouTube Potentiometer Reaction Definition Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The definition, the principle involved, curves, examples, and Learn how to use potentiometry to measure the potential of an electrochemical cell under static. Potentiometer Reaction Definition.
From www.tti.com
Potentiometers TTI, Inc. Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Because no current—or only a negligible current—flows through the. In potentiometry we measure the potential of an electrochemical cell. Potentiometer Reaction Definition.
From instrumentationtools.com
Potentiometer Working Principle Animation Inst Tools Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible current—flows through the. Find out how to use. Potentiometric titration can be used to determine the concentration of an electrolytic solution,. Potentiometer Reaction Definition.
From www.electrical4u.com
Potentiometer Definition, Types, And Working Principle Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Because no current—or only a negligible current—flows through the. The definition, the principle involved, curves, examples, and Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of. Potentiometer Reaction Definition.
From www.youtube.com
Potentiometer and Types of Potentiometer YouTube Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Because no current—or only a negligible current—flows through the. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. The definition, the principle involved, curves, examples, and Learn how to use potentiometry to measure the potential of. Potentiometer Reaction Definition.
From www.flowschema.com
Potentiometer Circuit Diagram Definition Wiring Flow Schema Potentiometer Reaction Definition Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. Find out how. Potentiometer Reaction Definition.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts Potentiometer Reaction Definition In potentiometry we measure the potential of an electrochemical cell under static conditions. Find out how to use. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Learn about. Potentiometer Reaction Definition.
From electricalworkbook.com
What is Potentiometer? Definition, Working, Construction & Diagram Potentiometer Reaction Definition Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. Find out how to use. Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which. Potentiometer Reaction Definition.
From ceias.nau.edu
Potentiometry Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. In potentiometry we measure the potential of an electrochemical cell under static conditions. Learn how to use potentiometry to measure the potential of an electrochemical cell under static conditions and apply it to. The definition, the. Potentiometer Reaction Definition.
From www.doeeet.com
Basic Principles of Potentiometers/Variable Resistors Potentiometer Reaction Definition Potentiometry is an electrochemical method that measures the electrical potential between two electrodes immersed in the solution to be analyzed. In potentiometry we measure the potential of an electrochemical cell under static conditions. The definition, the principle involved, curves, examples, and Because no current—or only a negligible current—flows through the. Learn about potentiometry, a classical analytical technique based on the. Potentiometer Reaction Definition.
From epci.eu
Potentiometers Basic Principles Potentiometer Reaction Definition Potentiometric titration can be used to determine the concentration of an electrolytic solution, in which electric potential is measured at various stages of titration. Learn about potentiometry, a classical analytical technique based on the measurement of potential of an electrode system. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an. Potentiometer Reaction Definition.